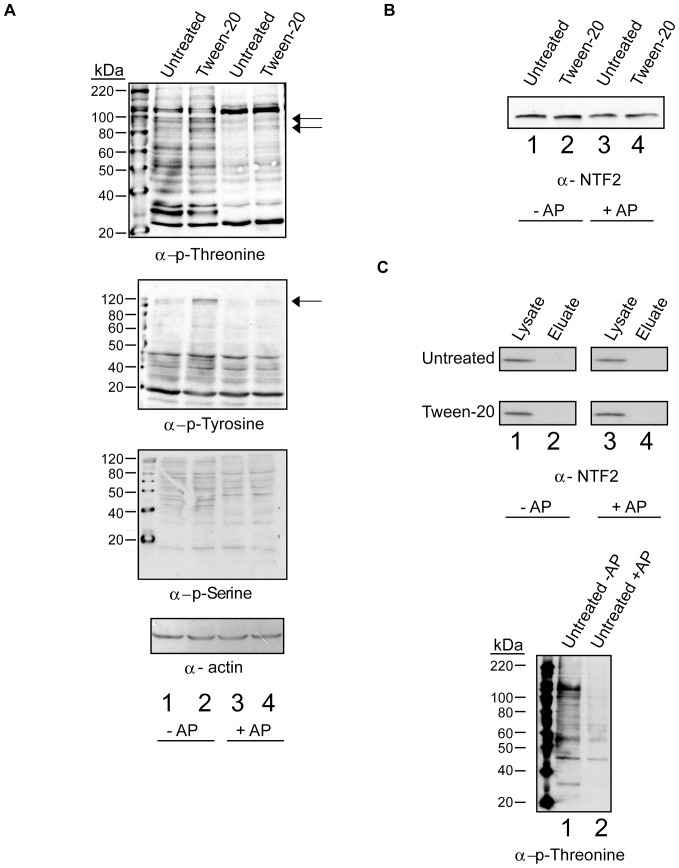
Figure 12. Tween-20 causes an increase in the level of protein phosphorylation at tyrosine and threonine residues, but does not appear to cause modification of NTF2.
(A) HeLa cells were incubated in serum-free DMEM without (Untreated) or with 150 µM Tween-20 (Tween-20) for 4 h. The cells were washed and lysed in the presence of sodium fluoride and sodium orthovanadate (Lanes 1 and 2, -AP) or lysed in the absence of phosphatase inhibitors (Lanes 3 and 4, +AP). The cells lysed without phosphatase inhibitors were incubated with alkaline phosphatase for 30 min at 37°C. Following the incubation, 40 µg of each lysate was separated on 10% gels by SDS-PAGE followed by Western blot analysis to monitor the levels of protein phosphorylation at threonine (first row), tyrosine (second row) and serine (third row) residues or actin level (fourth row). Arrows indicate bands of increased phosphorylation. (B) The lysate (40 µg) prepared as in (A) was separated by SDS-PAGE on a 15% gel followed by Western blot analysis to monitor the mobility of NTF2. The relative mobility of NTF2 was monitored in lysate prepared from Untreated (lanes 1 and 3) and Tween-20 (lanes 2 and 4) treated cells. (C) Lysates treated as in (A) were subjected to chromatography using Phostag™-agarose. The resins were washed and bound proteins were eluted using sodium phosphate containing buffer. Total cell lysate (20 µg) (lanes 1and 3, left) and eluted proteins were subjected to Western blot analysis. NTF2 was detected using α-NTF2 (left) and phospho-Thr proteins (lanes 1 and 2, right) were detected with anti-phospho-Thr antibodies.