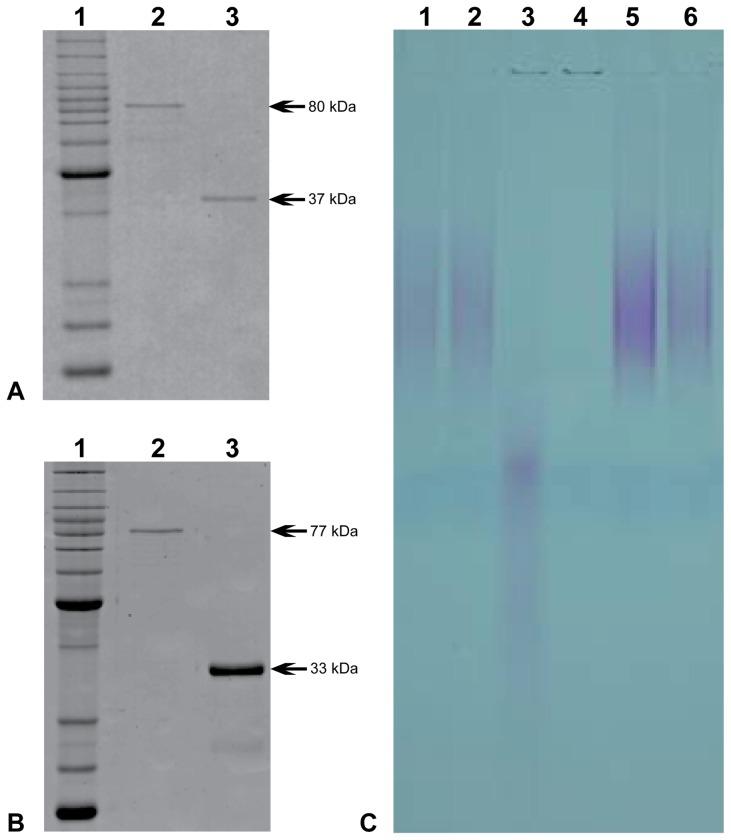
Figure 1. Purification and exopolyphosphatase activities of MTB-PPX1 and Rv1026.
Panel A: SDS-polyacrylamide gel of purified recombinant MTB-PPX1 (Rv0496) protein. Lane 1: protein ladder (BenchMark Protein from Invitrogen); lane 2, N-terminal Maltose Binding Protein (MBP)-MTB-PPX1 fusion protein (predicted molecular weight of 80 kDa); lane 3, untagged MTB-PPX1 (37 kDa; MBP-fusion removed using Factor Xa protease). Panel B: SDS-polyacrylamide gel of purified recombinant Rv1026 protein. Lane 1: protein ladder; lane 2: MBP-Rv1026 (77 kDa); lane 3: untagged Rv1026 (33 kDa). Panel C. Poly-P polyacrylamide gel showing exopolyphosphatase activities of MBP, MTB-PPX1, Rv1026 and E. coli GPP proteins. Reaction mixtures (100 µl) containing protein (5 µg), poly-P130 (0.1 mM), KCl (25 mM), with/without MnCl2 (1 mM) in HEPES buffer (50 mM, pH 6.8), were incubated for 1 hour at 37°C and analyzed on TBE 12% polyacrylamide gels. Lane 1: maltose binding protein (MBP; negative control); lane 2: MTB-PPX1, reaction without MnCl2; lane 3: MTB-PPX1; lane 4: E. coli GPP (EC-GPP); lane 5: Rv1026; lane 6: no protein added.