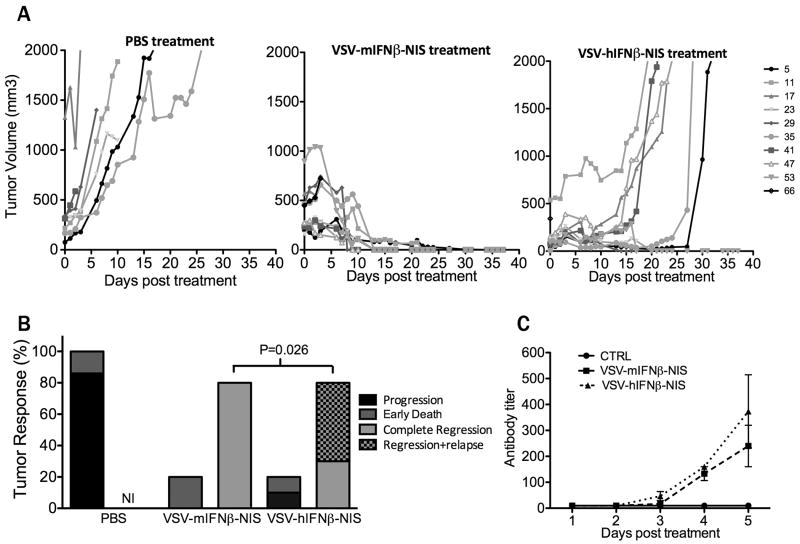
Figure 4. Therapeutic efficacy of systemically administered VSV-IFNβ-NIS.
Mice bearing subcutaneous 5TGM1 tumors were treated with a single intravenous dose of (i) PBS, (ii) VSV-mIFNβ-NIS or (iii) VSV-hIFNβ-NIS. (A) Tumor burden was measured by serial caliper measurements which were used to calculate tumor volume over time. (B) Tumor responses are categorized as tumor progression, early death (≤ 3 days post treatment), complete tumor regression, or regression followed by relapse. NI: no incidence. Statistical difference in rate of tumor relapse within mice with sustained tumor regression was measured by Fischer Exact test indicating significantly higher rate of tumor relapse in VSV-hIFNβ-NIS treated mice vs. VSV-mIFNβ-NIS treated mice (P=0.026). (C) Generation of VSV neutralizing antibodies is measured in serum of PBS treated (n=2) and VSV-IFNβ-NIS treated (n=3 for each virus) mice in the first 5 days post treatment and plotted as the minimum fold dilution that protects BHK cells from infection with 500 TCID50 VSV-GFP.