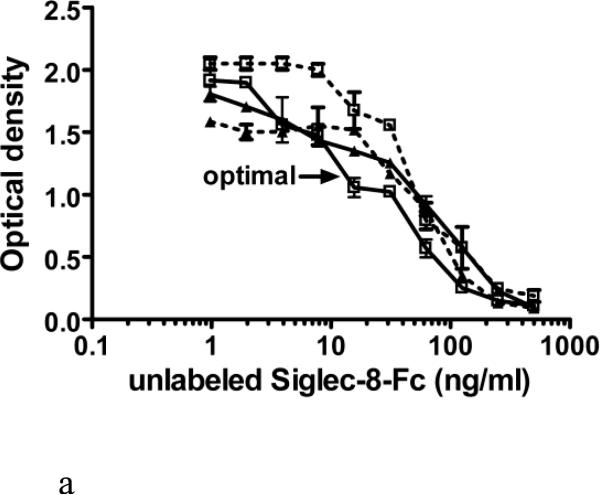
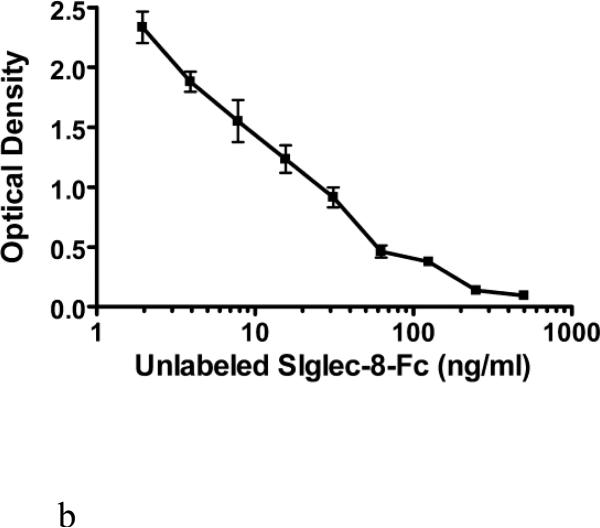
Figure 3.
Further fine optimization of the sSiglec-8 ELISA (Panel A) and validation of the selected assay conditions in replicate experiments (Panel B). Panel A shows sequential addition (30 min) of unlabeled Siglec-8-Fc (1-500 ng/ml) followed by a 1 hour incubation of Siglec-8-Fc biotin (30 ng/ml, solid lines or 40 ng/ml, dashed lines) without washing into a plate coated with 2C4 mAb at either 2 μg/ml (squares) or 2.5 μg/ml (triangles). The designation of “optimal” in Panel A refers to the best assay conditions, namely sequential addition of unlabeled Siglec-8-Fc, followed by addition of 30 ng/ml Siglec-8-Fc biotin, using 2 μg/ml of mAb 2C4 as the capture reagent, the conditions used to generate the averaged data (n=3, mean ± SD) shown in Panel B.