Abstract
The environmental factors that contribute to the development of autoimmune diseases are largely unknown. Endemic pemphigus foliaceus in humans, known as Fogo Selvagem (FS) in Brazil, is mediated by pathogenic IgG4 autoantibodies against desmoglein1 (Dsg1). Clusters of FS overlap with those of leishmaniasis, a disease transmitted by sand fly (Lutzomyia longipalpis) bites. In this study we show that salivary antigens from the sand fly, and specifically the LJM11 salivary protein, are recognized by FS antibodies. Anti-Dsg1 monoclonal autoantibodies derived from FS patients also cross-react with LJM11. Mice immunized with LJM11 generate anti-Dsg1 antibodies. Thus, insect bites may deliver salivary antigens that initiate a cross-reactive IgG4 antibody response in genetically susceptible individuals and lead to subsequent FS. Our findings establish a clear relationship between an environmental, non-infectious antigen and the development of potentially pathogenic autoantibodies in an autoimmune disease.
It is an accepted assumption that the interaction of unknown environmental factors with susceptibility genes of the host ensues the immune system to react to self-antigens causing an spectrum of autoimmune diseases (1). The common thread amongst autoimmune diseases is the obscure etiology. Human organ specific autoimmune diseases targeting the skin comprise the pemphigus group, where pathogenic IgG4-restricted, anti-epidermal autoantibodies cause epidermal cell detachment that leads to blister formation (2). The antigen recognized by these autoantibodies in PF is Dsg1. The idiopathic, non-endemic form of PF is known worldwide, whereas an endemic variety, FS is seen in certain regions of subtropical Brazil (3). FS shows similar clinical, histological and immunological features to non-endemic PF, except for the unique epidemiology of FS. A case-control epidemiological study of FS in Brazil suggested that certain living conditions and exposure to hematophagous insect bites were risk factors of FS (4). Exposure to bites of three insects is suspected to be linked to FS, Lutzomyia longipalpis (sand flies), reduviids (kissing bugs) and simuliids (black flies). They are vectors of leishmaniasis, Chagas disease and onchocerciasis respectively. Moreover, the sera of a large number of these patients possess anti-Dsg1 autoantibodies (5).
A recent study has demonstrated that not only IgM and IgG4 anti-Dsg1 autoantibodies are detected in the sera of FS but also IgE (6). It is remarkable that IgG4 anti-Dsg1 autoantibodies are restricted and pathogenic in FS, however, it is completely unknown the mechanisms involved in the emergence of these autoantibodies. The endemic nature of FS and the circumstantial evidence presented above allow us to test the hypothesis that salivary gland antigens from hematophagous insects are the source of sensitizing antigen that triggers the autoimmune disease in FS. We selected a well-defined system provided by Lutzomyia longipalpis, where the salivary gland proteins are well characterized (7, 8). In this investigation we show that IgG4 and IgE autoantibodies from FS sera recognized salivary gland antigens from Lutzomyia longipalpis (SGLL). The major SGLL antigenic component recognized by FS sera is LJM11. Additionally, sera from mice immunized with LJM11 also recognize human recombinant Dsg1. These results strongly support the notion that LJM11 induces cross-reactive antibodies in FS patients and experimental animals. This is the first evidence that a non-infectious agent may trigger a human autoimmune disease via molecular mimicry.
Materials and Methods
Serum samples and anti-Dsg1 monoclonal antibodies from FS patients
FS sera (N = 45) and two IgG4 anti-Dsg1 monoclonal antibodies (4E4 and 2D11) derived from FS patients (9), were used. Sera from healthy donors (n = 43) from the University of North Carolina blood bank were included as controls (HC-UNC). Ten sera from normal donors living in Brazilian endemic areas of FS were also included in some of the studies (HC-endemic). This study was approved by the Institutional review boards from universities of North Carolina, Chapel Hill and Sao Paulo, Brazil. The H and L chains of 4E4 and 2D11 (9) were cloned into pComb3XSS vector and expressed in Top10 F’ cells (10). A GST-tag was introduced to the 4E4 construct to increase the solubility of the recombinant 4E4 scFv, and 4E4-GST scFv was produced and purified by Genscript (Piscataway, NJ). The 4E4-GST scFv was not pathogenic when tested by passive transfer into neonatal mice (2) and the dispase assay (11) using concentrations up to 30ug/dose and 5ug/ml of the antibody respectively.
Recombinant Human Dsg1, Sand fly salivary gland extract, and sand fly salivary proteins
Recombinant Dsg1 was generated and purified as described (12). Salivary gland extracts from Lutzomyia longipalpis (SGLL) and SGLL proteins LJM11, LJM17, and LJL143 were generated at the Laboratory of Malaria and Vector Research, NIAID by Valenzuela (8, 13).
ELISA
IgE and IgG4 anti-Dsg1 and anti-SGLL ELISAs were conducted as described in previous communications (6, 9, 14). The ELISA assay to detect IgG4 antibody activity against LJM11, LJM17 and LJL143 SGLL proteins was also conducted as above with some modifications. Stripwell Microplates (Corning, Lowell, MA) were coated with 50ng/well with either LJM11, or LJM17, or LJL143 proteins. A 1:100 dilution of each of FS sera or scFv 4E4 and 2D11 anti-Dsg1 monoclonal antibodies (50ng/ml) was added and incubated. The bound IgG4 antibodies from serum samples or scFv antibodies were detected with anti-human IgG4 HRP (Zymed, San Francisco, CA) or anti-HA HRP conjugate (Roche, Indianapolis, IN), respectively. Anti-Dsg1 ELISA using SGLL recombinant proteins immunized mouse sera was conducted according to regular anti-Dsg1 ELISA described above with following modification. The mouse sera was diluted 1:500 and goat anti-mouse IgG (Fc fraction) HRP conjugate (Jackson ImmunoResearch, Carlsbad, CA) was used to detect anti-Dsg1 antibodies from mouse sera.
Immunoprecipitation (IP)
Pan mouse IgG magnetic Dynabead (Invitrogen, Carlsbad, CA) was used for the IP according to manufacturer’s instructions with modifications. Mouse anti-human IgG4 and Mouse anti-GST tag monoclonal antibodies (Zymed, San Francisco, CA) were used for the IP of sand fly salivary gland proteins LJM11, LJM17, and LJL143. All IPs were proceeded in the TBS buffer containing 0.5% Tween-20 and 5mM CaCl2 (TBS-T-Ca), plus 0.1% BSA. First the Dynabeads (20μl of slurry) were blocked with 0.1% BSA in TBS-T-Ca for 1 hour at room temperature (RT) and incubated with mouse anti-GST (for 4E4-GST) or mouse anti-human IgG4 (for serum samples) for 1 hour at RT, and then 4E4-GST or human serum samples for 2 hours at RT. Finally Dynabeads were incubated with LJM11, LJM17, and LJL143, respectively for 2 hours at RT. For IP using SGLL recombinant protein immunized mouse sera, 2μl of each mouse serum samples were incubated with 450μl recombinant Dsg1 cell culture supernatant for 2 hours at RT and then with 20μl of Dynabead slurry for 1 hour. Each IP sample was subjected to SDS-PAGE and subsequent Western Blot. The membranes were probed with anti-His HRP (Qiagen, Valencia, CA) and the bands revealed with chemiluminescent substrate (Pierce, Rockford, IL).
Statistical Analysis
Groups were compared by t-test. Correlation analysis was by the Pearson correlation.
Results and Discussion
FS patients have IgE and IgG4 antibodies against Lutzomyia longipalpis salivary gland antigens
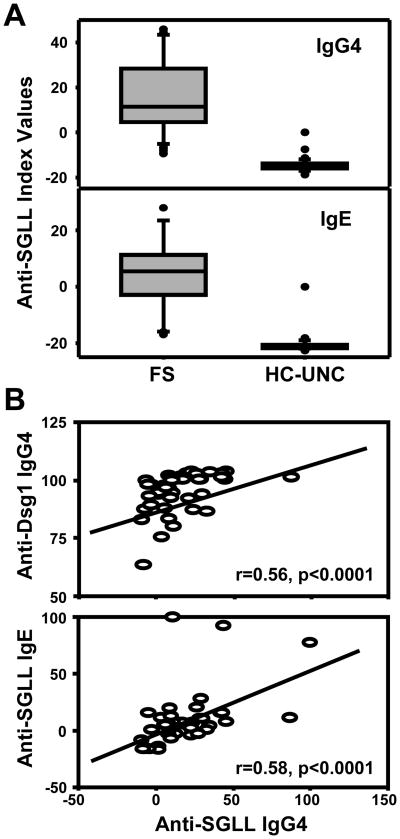
We have recently reported that FS patients possess significantly higher levels of IgE and IgG4 anti-Dsg1 antibodies than control groups from non-endemic areas of Brazil and US (6). It is possible that generation of these anti-Dsg1 autoantibodies maybe secondary to exposure and sensitization to an environmental antigen(s). The sera from FS patients, and healthy controls from US (HC-UNC) were tested for IgE and IgG4 antibody activity against SGLL by ELISA. As shown in Figure 1A, the index values of IgE and IgG4 anti-SGLL antibodies are significantly higher in FS patients (n=45) than HC-UNC controls (n=43) (p<0.001). The IgG4 anti-SGLL antibody response in the FS group was significantly correlated with both the IgG4 anti-Dsg1 [Pearson correlation, (r=0.56, p<0.0001) and IgE anti-SGLL (r=0.58, p<0.0001)] (Figure 1B). These findings strongly suggest that the generation of potentially pathogenic IgG4 anti-Dsg1 in FS may be associated with the antigenic stimulation produced by salivary antigens from hematophagous insects.
Figure 1.
FS patients have high levels of anti-SGLL IgG4 and IgE antibodies. (A). Boxplot analysis of the IgG4 and IgE responses in FS patients (n=45) and UNC healthy controls (n=43) against SGLL antigens by ELISA. The index values are higher in the FS group than the HC-UNC group. (B). Pearson correlation analysis between the IgG4 anti-SGLL and the IgG4 anti-Dsg1 responses (upper panel) and the IgE anti-SGLL and IgG4 anti-SGLL responses (lower panel) in the same donors of the FS group.
The cross-reactivity of anti-Dsg1 antibodies to SGLL
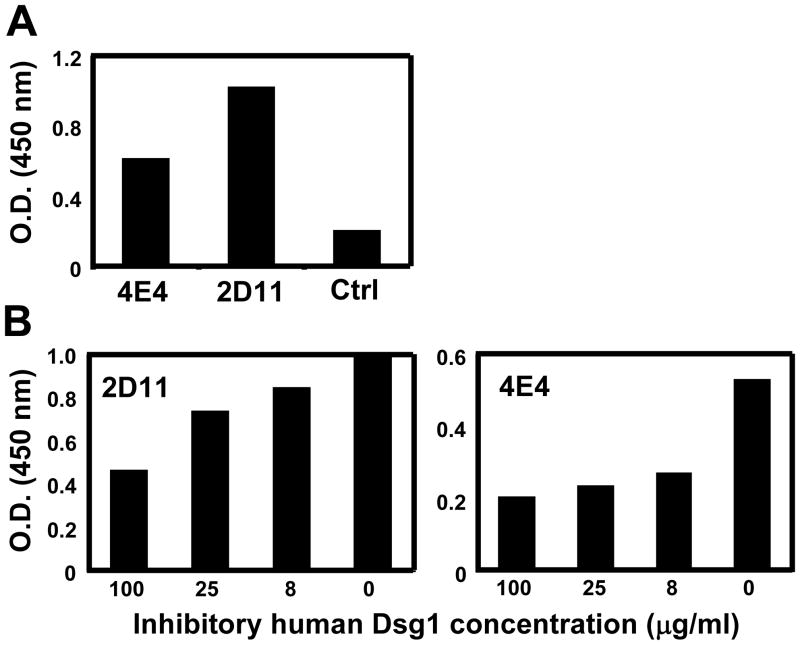
To determine whether the anti-SGLL activity from FS sera is due to the cross-reactivity of anti-Dsg1 autoantibodies, ELISA was performed with two scFv IgG4 monoclonal anti-Dsg1 antibodies derived from two FS patients (9) against SGLL antigens. As shown in Figure 2A, both scFv recognize SGLL. To confirm the cross-reactivity of the two monoclonal anti-Dsg1 autoantibodies with anti-SGLL antibodies, a competition ELISA assay was employed using human recombinant Dsg1 as an inhibitor of the binding of anti-SGLL antibodies to immobilized SGLL antigen. As shown in Figure 2B, binding of both monoclonal antibodies to SGLL are inhibited in a dose dependent manner by soluble Dsg1. These findings suggest that the anti-SGLL antibody activity in FS patients is, at least in part, due to cross-reactivity of the anti-Dsg1 autoantibodies.
Figure 2.
The cross-reactivity of two IgG4 anti-Dsg1 monoclonal antibodies from FS to SGLL by ELISA. (A). Both IgG4 anti-Dsg1 monoclonal antibodies (scFv 4E4 and scFv 2D11) react to SGLL. (B). The binding of both monoclonal antibodies, 2D11 (left panel) and 4E4 (right panel) to SGLL is inhibited by Dsg1 in a concentration dependent manner. These results were repeated three times with similar results.
The LJM11 major immunogenic component of SGLL is recognized by FS sera and IgG4 monoclonal anti-Dsg1 antibodies
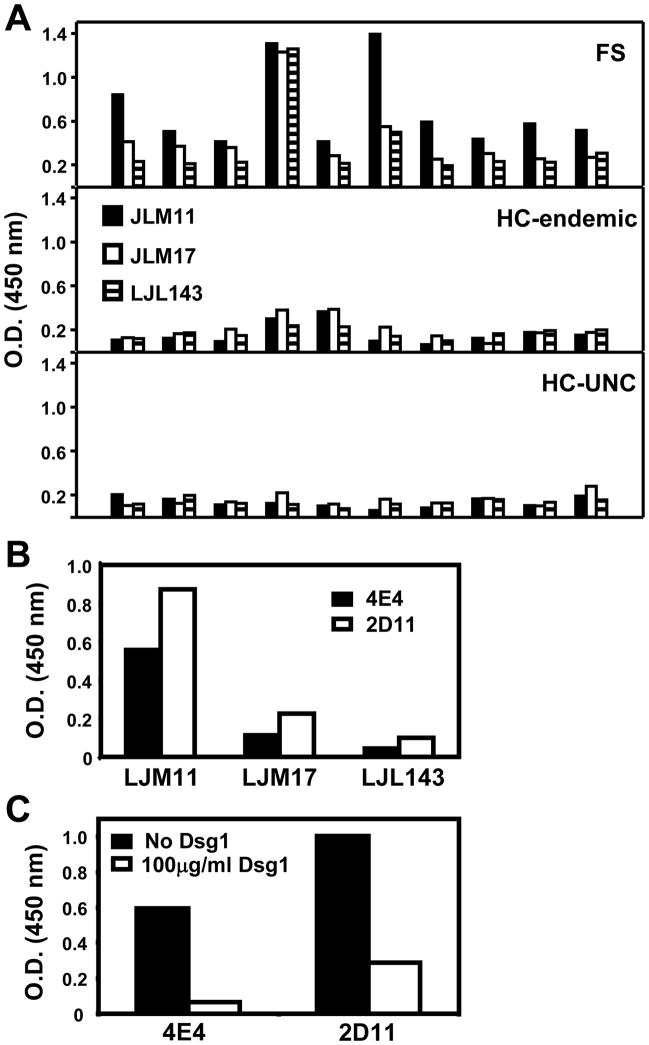
A large number of secreted proteins are present in Lutzomyia longipalpis saliva (7, 8, 13) and, LJM11 and LJM17 are the two main antigens that incite antibody responses in humans. Other salivary antigens such as the LJL143 are weakly recognized by humans (13). These three recombinant proteins were chosen to determine whether they are recognized by IgG4 antibodies from FS patients and normal controls from either FS endemic regions (HC-endemic) or from non-FS endemic region (HC-UNC). Ten serum samples from each group were randomly selected and tested. As shown in Figure 3A, we found that the reactivity of FS sera was consistently much stronger to LJM11 than to LJM17 and LJL143. In addition, the level of anti-LJM11 antibodies from FS is significantly higher than healthy donors from HC-endemic (t-test, p=0.00029) and HC-UNC (t-test, p=0.00013). These results show that IgG4 antibodies from FS sera chiefly recognize the LJM11 component of SGLL.
Figure 3.
IgG4 antibodies from FS patients and two IgG4 monoclonal anti-Dsg1 antibodies derived from FS patients recognize LJM11, a protein component from SGLL. (A). The reactivity of ten random-selected FS sera (top panel), is higher than donors from the HC-endemic (middle panel) and HC-UNC donors (bottom panel) when tested by ELISA with three SGLL main proteins, LJM11, LJM17, and LJL143. The reactivity is mainly with the LJM11 protein. The differences between the HC-endemic and HC-UNC by the student-t test is not significant (p=0.3800). (B) The 4E4 and 2D11 IgG4 monoclonal anti-Dsg1 antibodies also recognize the LJM11 component from SGLL. (C). The binding of both anti-Dsg1 monoclonal antibodies to LJM11 is inhibited by Dsg1 protein.
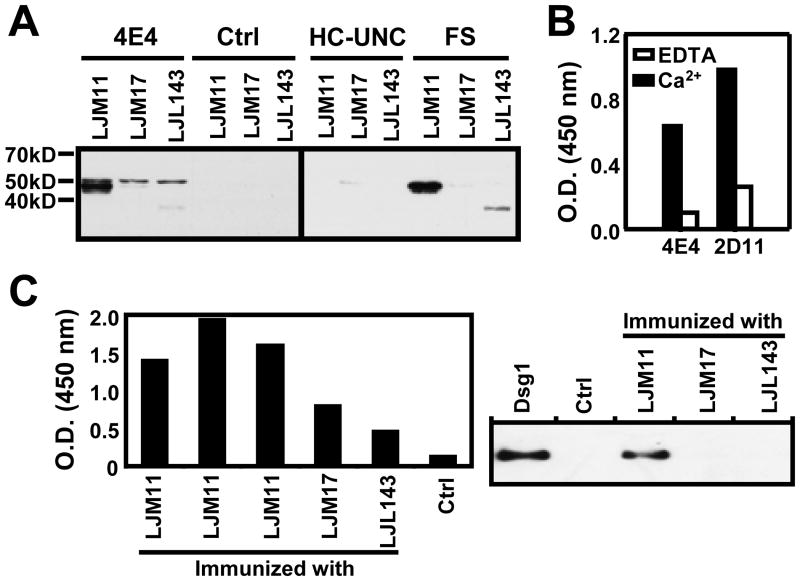
We next tested the reactivity of 4E4 and 2D11 IgG4 anti-Dsg1 monoclonal antibodies against LJM11, LJM17 and LJL143. As shown in Figure 3B, both monoclonal antibodies bind strongly to LJM11, but weakly to LJM17 and LJL143. These findings are similar to those observed when testing FS sera. To further confirm that these two monoclonal antibodies cross-react to LJM11, an ELISA inhibition assay was conducted. A doses of 100μg/ml of recombinant Dsg1 incubated with these antibodies was able to inhibit 90% and 70% of the bindings of 4E4 and 2D11 to LJM11 respectively (Figure 3C). In addition, IP using monoclonal antibody 4E4, and FS patient serum show that both react strongly to LJM11 (~43kD), but weakly to LJM17 (~45kD) and LJL143 (~34kD) (Figure 4A). These findings suggest that LJM11 is the main component of SGLL that triggers the IgG4 immune response in humans that cross-react with Dsg1 autoantigen.
Figure 4.
FS autoantibodies recognize LJM11 and sera from mice immunized with LJM11 bind human Dsg1. (A). The 4E4 anti-Dsg1 monoclonal antibody (lanes 1,2,3), and control samples without 4E4 (lanes 4,5,6) [left panel], HC-UNC (lanes 7,8,9) and FS serum (lanes 10, 11, 12) [right panel] were tested by IP against LJM11, LJM17 and LJL143 antigens. The LJM11 heavy band is precipitated by 4E4 anti-Dsg1 monoclonal antibody and FS serum. (B). The reactions of 4E4 and 2D11 anti-Dsg1 monoclonal antibodies with LJM11 by ELISA in the presence of 5mM Ca2+ (black columns) are significantly inhibited in the presence of 5mM EDTA (white columns). (C). Three sera from mice immunized with LJM11, show strong reactivity to human Dsg1 by ELISA as compared with sera from mice immunized with LJM17 and LJL143 and control mice (left panel). The right panel shows human Dsg1 (lane 1), a control murine serum (lane 2), mouse anti-LJM1 (lane 3), mouse anti-LJM17 (lane 4) and mouse anti-LJL143 (lane 5) tested against human Dsg1 by IP. The only serum that immunoprecipitates human Dsg1 is from mouse anti-LJM11.
The binding of anti-Dsg1 monoclonal antibodies to LJM11 is conformational and Ca2+ dependent
Both 4E4 and 2D11 anti-Dsg1 monoclonal antibody failed to react with denatured LJM11, LJM17, and LJL143 by Western blot analysis (data not shown), suggesting that 4E4 antibody binding to the SGLL is conformational. Since binding of pathogenic autoantibodies to Dsg1 is conformational and Ca2+ dependent (15), we tested the reactivity of these two antibodies to LJM11 in the presence of either Ca2+ or EDTA. As shown in Figure 4B the binding of these monoclonal antibodies from FS patients to LJM11 is also Ca2+-dependent. These results suggest that the epitopes recognized by FS sera on SGLL, like those on Dsg1, are Ca2+ dependent and conformational.
Mice immunized with LJM11 produce anti-Dsg1 antibodies
If SGLL proteins introduced by sand fly bites induce antibodies that cross-react to both human Dsg1 and LJM11 in humans, it is expected that mice immunize with LJM11 also generate cross-reactive antibodies to human Dsg1. Anti-Dsg1 reactivity of the sera from these mice and control mouse were test by ELISA. As shown in Figure 4C (left panel), sera from all three mice immunized with LJM11 strongly react to human Dsg1. The Dsg1 reactivity of these mouse sera was also tested by IP. As shown in Figure 4C (right panel), the serum from a mouse immunized with LJM11 antigen also immunoprecipitates human Dsg1. Control serum and sera from mice immunized with LJM17 and LJL143 antigens do not show reactivity. These findings further confirm that LJM11 is the major component in SGLL that induces the cross-reactive antibodies in both human FS patients and experimental mice.
It is unusual that a population of antibodies would develop cross-reactivity to two evolutionarily distant molecules, the human Dsg1 and a sand fly salivary gland component. There is no amino acid sequence similarity between Dsg1 and LJM11 (data not shown). The Ca2+-dependent interaction suggests that autoantibodies react with both molecules via conformational, but not linear, epitopes. This could explain how two evolutionary distant molecules can both be recognized by cross-reactive antibodies since conformational epitopes of both molecules may be the same even though their linear structures do not show significant homology.
Our findings are also in line with molecular mimicry as the mechanism for how environmental factors induce autoimmune diseases (1, 16). Unlike infectious agents that induce robust IgG immune response, the low dose of the antigens and the route of the exposure (skin) introduced by insect bites predictably induces an IgE response (17). It is known that endemic regions of FS in Brazil are heavily infested of sand flies, and individuals living in these areas are constantly exposed to these pests. It is likely that these individuals mount IgE and IgG4 responses to salivary antigens such as the LJM11 protein. Similar antibody responses have been described in people undergoing constant bee stings or during immunotherapy of allergic patients (18–21). It would be expected that those genetically predisposed individuals (22) living in endemic areas of FS are constantly exposed to sand fly bites (and be sensitized to salivary antigens, including the LJM11 protein), thus generating IgG4 and IgE anti-SGLL antibodies which, as shown in this investigation, may cross-react with unique epitopes on the ectodomain of human Dsg1. Epitope spreading, as previously reported (23), may be the underlying mechanism that leads to the generation of a more diverse autoantibody response in FS. The cross-reactive antibodies may comprise a complex population of non-pathogenic and perhaps pathogenic antibodies. In this context, it would be feasible that certain FS IgG4 autoantibodies may exhibit distinct epitope specificity with those IgG4 anti-SGLL antibodies that cross-react with human Dsg1. It is clear however, that testing the pathogenicity of these cross-reactive antibodies is open for further investigations. In summary, this investigation provides the first direct evidence that a non-infectious environmental agent may play a significant role in the initiation of an autoimmune disease via molecular mimicry.
Acknowledgments
This work was supported in part by the NIH grants R01 AR30281, R01 AR32599 (to LAD), and K01 AR056378 (to YQ). Dr. Qian's research was also supported by a Dermatology Foundation Research Award and an American Skin Association Alice P. Melly research grant.
Abbreviations used in this paper
- Dsg1
desmoglein 1
- FS
Fogo Selvagem
- HPLC
high-performance liquid chromatography
- PF
pemphigus foliaceus
- SGLL
salivary gland antigens from Lutzomyia longipalpis
References
- 1.Miller FW. Environmental agents and autoimmune diseases. Adv Exp Med Biol. 2011;711:61–81. doi: 10.1007/978-1-4419-8216-2_6. [DOI] [PubMed] [Google Scholar]
- 2.Rock B, Martins CR, Theofilopoulos AN, Balderas RS, Anhalt GJ, Labib RS, Futamura S, Rivitti EA, Diaz LA. The pathogenic effect of IgG4 autoantibodies in endemic pemphigus foliaceus (fogo selvagem) N Engl J Med. 1989;320:1463–1469. doi: 10.1056/NEJM198906013202206. [DOI] [PubMed] [Google Scholar]
- 3.Diaz LA, Sampaio SA, Rivitti EA, Martins CR, Cunha PR, Lombardi C, Almeida FA, Castro RM, Macca ML, Lavrado C, et al. Endemic pemphigus foliaceus (Fogo Selvagem): II. Current and historic epidemiologic studies. J Invest Dermatol. 1989;92:4–12. doi: 10.1111/1523-1747.ep13070394. [DOI] [PubMed] [Google Scholar]
- 4.Aoki V, Millikan RC, Rivitti EA, Hans-Filho G, Eaton DP, Warren SJ, Li N, Hilario-Vargas J, Hoffmann RG, Diaz LA. Environmental risk factors in endemic pemphigus foliaceus (fogo selvagem) J Investig Dermatol Symp Proc. 2004;9:34–40. doi: 10.1111/j.1087-0024.2004.00833.x. [DOI] [PubMed] [Google Scholar]
- 5.Diaz LA, Arteaga LA, Hilario-Vargas J, Valenzuela JG, Li N, Warren S, Aoki V, Hans-Filho G, Eaton D, dos Santos V, Nutman TB, de Mayolo AA, Qaqish BF, Sampaio SA, Rivitti EA. Anti-desmoglein-1 antibodies in onchocerciasis, leishmaniasis and Chagas disease suggest a possible etiological link to Fogo selvagem. J Invest Dermatol. 2004;123:1045–1051. doi: 10.1111/j.0022-202X.2004.23438.x. [DOI] [PubMed] [Google Scholar]
- 6.Qian Y, Prisayanh P, Andraca E, Qaqish BF, Aoki V, Hans-Filhio G, Rivitti EA, Diaz LA. IgE, IgM, and IgG4 anti-desmoglein 1 autoantibody profile in endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2011;131:985–987. doi: 10.1038/jid.2010.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valenzuela JG, Garfield M, Rowton ED, Pham VM. Identification of the most abundant secreted proteins from the salivary glands of the sand fly Lutzomyia longipalpis, vector of Leishmania chagasi. J Exp Biol. 2004;207:3717–3729. doi: 10.1242/jeb.01185. [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Oliveira F, Chang BW, Collin N, Gomes R, Teixeira C, Reynoso D, My Pham V, Elnaiem DE, Kamhawi S, Ribeiro JM, Valenzuela JG, Andersen JF. Structure and function of a “yellow” protein from saliva of the sand fly Lutzomyia longipalpis that confers protective immunity against Leishmania major infection. J Biol Chem. 2011;286:32383–32393. doi: 10.1074/jbc.M111.268904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian Y, Clarke SH, Aoki V, Hans-Filhio G, Rivitti EA, Diaz LA. Antigen selection of anti-DSG1 autoantibodies during and before the onset of endemic pemphigus foliaceus. J Invest Dermatol. 2009;129:2823–2834. doi: 10.1038/jid.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barbas CFI, Burton DR, Scott JK, Silverman GJ. Phage display: a laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York, USA: 2001. [Google Scholar]
- 11.Ishii K, Harada R, Matsuo I, Shirakata Y, Hashimoto K, Amagai M. In vitro keratinocyte dissociation assay for evaluation of the pathogenicity of anti-desmoglein 3 IgG autoantibodies in pemphigus vulgaris. J Invest Dermatol. 2005;124:939–946. doi: 10.1111/j.0022-202X.2005.23714.x. [DOI] [PubMed] [Google Scholar]
- 12.Ding X, Diaz LA, Fairley JA, Giudice GJ, Liu Z. The anti-desmoglein 1 autoantibodies in pemphigus vulgaris sera are pathogenic. J Invest Dermatol. 1999;112:739–743. doi: 10.1046/j.1523-1747.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 13.Teixeira C, Gomes R, Collin N, Reynoso D, Jochim R, Oliveira F, Seitz A, Elnaiem DE, Caldas A, de Souza AP, Brodskyn CI, de Oliveira CI, Mendonca I, Costa CH, Volf P, Barral A, Kamhawi S, Valenzuela JG. Discovery of markers of exposure specific to bites of Lutzomyia longipalpis, the vector of Leishmania infantum chagasi in Latin America. PLoS Negl Trop Dis. 2010;4:e638. doi: 10.1371/journal.pntd.0000638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qaqish BF, Prisayanh P, Qian Y, Andraca E, Li N, Aoki V, Hans-Filho G, dos Santos V, Rivitti EA, Diaz LA. Development of an IgG4-based predictor of endemic pemphigus foliaceus (fogo selvagem) J Invest Dermatol. 2009;129:110–118. doi: 10.1038/jid.2008.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amagai M, Ishii K, Hashimoto T, Gamou S, Shimizu N, Nishikawa T. Conformational epitopes of pemphigus antigens (Dsg1 and Dsg3) are calcium dependent and glycosylation independent. J Invest Dermatol. 1995;105:243–247. doi: 10.1111/1523-1747.ep12317587. [DOI] [PubMed] [Google Scholar]
- 16.Fujinami RS, von Herrath MG, Christen U, Whitton JL. Molecular mimicry, bystander activation, or viral persistence: infections and autoimmune disease. Clin Microbiol Rev. 2006;19:80–94. doi: 10.1128/CMR.19.1.80-94.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Platts-Mills TA, Woodfolk JA. Allergens and their role in the allergic immune response. Immunol Rev. 2011;242:51–68. doi: 10.1111/j.1600-065X.2011.01021.x. [DOI] [PubMed] [Google Scholar]
- 18.Gleich G, Zimmermann E, Henderson L, Yunginger J. Effect of immunotherapy on immunoglobulin E and immunoglobulin G antibodies to ragweed antigens: a six-year prospective study. J Allergy Clin Immunol. 1982;70:261–271. doi: 10.1016/0091-6749(82)90062-8. [DOI] [PubMed] [Google Scholar]
- 19.Golden D, Lawrence I, Hamilton R, Kagey-Sobotka A, Valentine M, Lichtenstein L. Clinical correlation of the venom-specific IgG antibody level during maintenance venom immunotherapy. J Allergy Clin Immunol. 1992;90:386–393. doi: 10.1016/s0091-6749(05)80019-3. [DOI] [PubMed] [Google Scholar]
- 20.Lichtenstein L, Holtzman N, Burnett L. A Quantitative in Vitro Study of the Chromatographic Distribution and Immunoglobulin Characteristics of Human Blocking Antibody. J Immunol. 1968;101:317. [PubMed] [Google Scholar]
- 21.Rossi R, Monasterolo G, Coco G, Silvestro L, Operti D. Evaluation of serum IgG4 antibodies specific to grass pollen allergen components in the follow up of allergic patients undergoing subcutaneous and sublingual immunotherapy. Vaccine. 2007;25:957–964. doi: 10.1016/j.vaccine.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 22.Moraes ME, Fernandez-Vina M, Lazaro A, Diaz LA, Hans-Filho G, Friedman H, Rivitti EA, Aoki V, Stastny P, Moraes JR. An epitope in the third hypervariable region of the DRB1 gene is involved in the susceptibility to endemic pemphigus foliaceus (fogo selvagem) in three different Brazilian populations. Tissue Antigens. 1997;49:35–40. doi: 10.1111/j.1399-0039.1997.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 23.Li N, Aoki V, Hans-Filho G, Rivitti EA, Diaz LA. The role of intramolecular epitope spreading in the pathogenesis of endemic pemphigus foliaceus (fogo selvagem) J Exp Med. 2003;197:1501–1510. doi: 10.1084/jem.20022031. [DOI] [PMC free article] [PubMed] [Google Scholar]