Abstract
Endothelial progenitor cells (EPCs) correspond to a population of cells with novel properties capable of angiogenesis and vasculogenesis, thus they are likely to display unique role in the reconstitution of the blood brain barrier (BBB) after stroke. Laboratory evidence supports safety and efficacy of cell therapy for stroke, with limited clinical trials recently initiated. This lab-to-clinic ascent of cell-based therapeutics has been aided by the establishment of consortium consisting of thought-leaders from academia, industry, National Institutes of Health (NIH) and the United States Food and Drug Administration (FDA). However, there remain unanswered questions prior to realization of large-scale application of cell transplantation in patients. This review article discusses translational challenges associated in cell therapy, emphasizing the need for optimizing both safety and efficacy profiles for advancing the clinical applications of EPC transplantation for stroke patients.
Keywords: Cerebral ischemia, stem cells, cell transplantation, translational research
INTRODUCTION
Stem cell-based therapeutics for stroke have recently commenced in the clinic [1-3]. This is a welcome relief for many stroke patients. Despite the advance in our scientific knowledge, stroke is still a major cause of death and disability worldwide. Accordingly, finding a novel treatment, which can be effective well beyond the acute 3-hour window after disease onset, is being heralded as a unique treatment regimen in the clinic. In 1998, the world’s first clinical trial of cell therapy in stroke was performed [4]. In recent years, the advancement of stem cell-based therapeutics from the laboratory to the clinic has been guided by research recommendations from STEPS (Stem Cell Therapeutics as an Emerging Paradigm for Stroke), a consortium of experts from the academia, industry, NIH and FDA. These STEPS guidelines are designed to enhance the safety and efficacy of stem cell-based therapeutics as we translate these novel treatments to stroke patients. We discuss here how endothelial progenitor cells (EPCs) can take advantage of STEPS as the cells move toward clinical application.
EPCS FOR CELL THERAPY
There are distinct stem cell populations in the bone marrow [5]. Hematopoietic stem cells (HSCs), mesenchymal stem cells (MSCs), EPCs and very small embryonic-like stem cells (VSELs) are some of these stem cells shown to commit toward neural lineage [6] though controversial, and have the ability to secrete growth factors [7-9]. A key chemoattractant process exploited by HSCs [11-13] for their mobilization and migration is the SDF-1/CXCR4 signaling pathway [14]. On the other hand, the plastic-adherent MSCs [15], which are phenotypically divergent from HSC, exert their therapeutic potential by their multiple lineage differentiation [16-20]. Although as noted above, the neuronal differentiation of MSCs has been challenged as the mechanism underlying the observed functional recovery in stroke [21-30]. An alternative mechanism of repair by cell therapy in stroke is the stimulation of resident stem cells. To this end, VSELs are detected in mature tissues [31-33] and initially migrate to the circulation and subsequently to the site of injury after an insult [31,34]. The recognition of stem cells (i.e., VSELs) in the circulatory system is paralleled by detection of EPCs in the vasculature [35-39]. Similar to VSELS, EPCs have been detected in adult tissues [40,41]. Transplanted EPCs honed to immature vasculature of damaged tissues following stroke [35], suggesting that they actively contribute to angiogenesis and vasculogenesis [35,36,39,41-43]. A unique advantage of EPCs over HSCs, MSCs and VSELs is their potential to repair the damaged neurovasculature. We posit that the reconstitution of the blood brain barrier (BBB) in stroke can be achieved by EPCs via their angiogenic and vasculogenic properties. Indeed, stroke patients arguably with damaged BBB have received EPC transplantation based on the cells’ reparative effects on the vasculature [44]. The subsequent sections discuss the translational caveats for advancing EPC therapy in stroke.
SAFETY AND EFFICACY PROFILING OF EPCS
The STEPS consortium, now on its third meeting, is designed to optimize safety and efficacy of stem cell-based therapeutics in stroke [3]. In this vein, the recognition that ischemic stroke has several subtypes necessitates the need for laboratory studies [45]. Stroke heterogeneity requires careful preclinical modeling, with the use of young and old animals, male and female animals, transient and permanent models in order to optimize the cell transplant regimen [1,3, 46-48]. Additionally, for safety concern the large animal with closer vasculature as humans may be a better model to address the toxicology readouts to eliminate the possibility that transplanted cells leads to microembolism. With this safety issue in mind, the different routes of cell delivery should also be critically assessed, which may reveal relegating this transplant approach to a certain stroke population, e.g., acute versus chronic stroke patiens. Altogether, the safety assessment needs to be conducted in parallel with efficacy readouts in order to fully assess the value of this novel treatment. Along this line, the safety of EPCs has been examined in the clinic [49]. Because these studies are open label trials, and consist mostly of descriptive data, and a small sample size, they warrant a careful examination of the safety data.
The investigation of EPCs in neurovascular disease in the clinic is limited to a few observational studies. Transient upregulation of CD34+ cells was seen acutely which waned over time [50], while there was a reduced detection of adherent cells in patients diagnosed with neurovascular injury compared to controls free of vascular disease [38]. With a small patient population, these discrepant results are expected. Accordingly, a similar scrutiny of EPC efficacy data is indicated.
STEPS REQUIREMENTS FOR EPC THERAPY IN STROKE
A basic STEPS requirement is the characterization of the donor cell [3]. As noted above, a panel of surface antigen markers and a molecular signature of stemness behavior, as well as expected function [42,51-54], have been employed to harvest EPCs [35,42,52, 53,55,56,57]. Recently, the AC133 surface marker has been utilized to isolate EPCs [42,54], and the co-labeling with known EPC-antigen labels [42,54,58]. In addition, CD34 positive EPCs have been shown to differentiate into mature endothelial cells [39,49,56]. Finally, functional profiling has also been performed to reveal EPC phenotypes [35,58-60].
CELL TRANSPLANT REGIMEN FOR EPCS
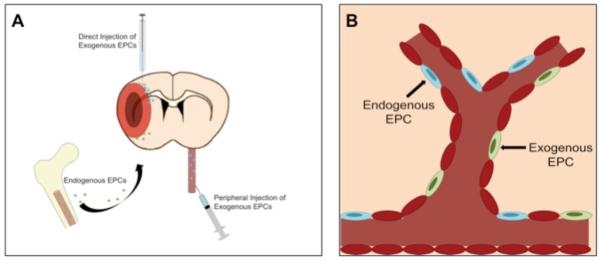
Here, a forward-looking approach in the preclinical study should reveal clinically relevant transplant regimen. A keen eye directed at the clinical stroke scenario will aid in expediting the clinical entry of cell therapy. A scenario involving stereotaxic transplantation of cells immediately after stroke would indicate the likely need for a neurosurgeon to administer the cells. On one hand, the demonstration that cell injection is possible through peripheral route would expect a patent chemoattractant pathway to guide the cells during the early state of the disease. In a similar fashion, the knowledge of mechanism of action will be critical to optimizing the transplant regimen. The putative vasculature-restorative feature of EPC implicates the need to better understand the timing of neurovascular damage that would allow robust functional outcome. In the end, the multiple technical issues (dose, route and timing of cell transplantation) and relevant clinical scenarios need to be an integral part of the experimental design for successful clinical translation of cell therapy in stroke Fig (1).
Fig (1).
EPC transplantation and BBB repair. Neurovascular repair is achieved via endogenous EPCs mobilized towards the ischemic brain, which can be enhanced by exogenous EPC delivery either from the periphery or direct intracerebral transplantation (Panel A). Following honing to the area of injured vasculature, the EPCs promote restoration of BBB, potentially affording anti-inflammatory effects then abrogating stroke-induced histopathological symptoms (Panel B).
FUNCTIONAL CHARACTERIZATION OF EPC TRANSPLANTS IN STROKE
A realm of motor deficits accompanies the rodent model of ischemic injury [60-64]. The recognition of the need to assess cognitive deficits has also been acknowledged because stroke presents with both motor and cognitive impairments in the clinic. A major caveat for assessing cognitive behaviors in experimental stroke is that the cognitive areas of the brain should be injured, indicating that not all stroke animals may be appropriate for cognitive testing. For both motor and cognitive tests, the need for long term testing (i.e., minimum of one month post-stroke) is required to reveal the stability of therapeutic outcomes. In view of EPCs’ unique features related to BBB repair, the choice of functional tests may benefit from combined evaluation of BBB repair as a mechanism underlying the functional recovery in stroke animals. In this case, a rationale and logical functional characterization of EPC transplantation will allow elucidation of cellular and molecular pathways of stem cell-based therapeutics. Indeed, the US FDA has been enthusiastic in recent years in obtaining insights into these signaling pathways. For EPC, it appears that its stroke target is the subsequent BBB breakdown after the initial ischemic event. Aberrant inflammatory responses following this ischemic event promote cell death processes, including BBB breakdown in turn allowing the CNS entry of more pro-inflammatory factors and altogether exacerbating the stroke pathology [65]. Although it is not clear, transplanted EPCs may restore the barrier’s integrity and function. Additional studies are warranted to reveal the role of EPCs in BBB restoration as a mechanism of action in stroke therapy. Imaging modalities such as functional magnetic resonance imaging may reveal not only graft survival, but also host tissue response (i.e., BBB status) after stroke and transplantation [66,67].
CONCLUSIONS
Cell-based stroke therapeutics using EPCs require a careful and systematic laboratory-to-clinic translational research. Preclinical studies need to pursue a rigorous set experiments designed to critically assess both safety and efficacy of EPCs. Moreover, the understanding of EPC mechanism of action will be key to optimizing EPC therapy in stroke. In particular, that EPCs are closely associated with angiogenesis and vasculogenesis implicate a novel mechanism of action involving EPCs’ critical role in BBB repair following stroke. Their mobilization and migration to the ischemic injury, both endogenously and following exogenous transplantation, concomitant with improved vasculature integrity, indicate their robust ability to abrogate BBB breakdown [68,69]. A combination of basic science research addressing the mechanism of action, and the aggressive translational platform harnessing close collaboration with academic and industry researchers, and NIH and FDA regulators should allow a safe and effective EPC transplant therapy for stroke.
ACKNOWLEDGEMENTS
The Borlongan Laboratory is supported by James and Esther King Foundation for Biomedical Research Program, USF Signature Program in Interdisciplinary Neuroscience, SanBio Inc., Celgene Cellular Therapeutics, KMPHC, NeuralStem Inc., NIH NINDS UO15U01NS055914-04, and NIH NINDS R01NS071956-01. CVB has patents and patent applications on cell therapy.
REFERENCES
- [1].Borlongan CV, Chopp M, Steinberg GK, et al. Potential of stem/progenitor cells in treating stroke: the missing steps in translating cell therapy from laboratory to clinic. Regen Med. 2008;3:249–50. doi: 10.2217/17460751.3.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Chopp M, Steinberg GK, Kondziolka D, et al. Who’s in favor of translational cell therapy for stroke: STEPS forward please? Cell Transplant. 2009;18:691–3. doi: 10.3727/096368909X470883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Stem Cell Therapies as an Emerging Paradigm in Stroke Participants. Stem Cell Therapies as an Emerging Paradigm in Stroke (STEPS): bridging basic and clinical science for cellular and neurogenic factor therapy in treating stroke. Stroke. 2009;40:510–15. doi: 10.1161/STROKEAHA.108.526863. [DOI] [PubMed] [Google Scholar]
- [4].Kondziolka D, Wechsler L, Goldstein S, et al. Transplantation of cultured human neuronal cells for patients with stroke. Neurology. 2000;55:565–9. doi: 10.1212/wnl.55.4.565. [DOI] [PubMed] [Google Scholar]
- [5].Herzog EL, Chai L, Krause DS. Plasticity of marrow-derived stem cells. Blood. 2003;102:3483–93. doi: 10.1182/blood-2003-05-1664. [DOI] [PubMed] [Google Scholar]
- [6].Munoz-Elias G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor function. Stem Cells. 2003;21:437–48. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- [7].Hara K, Yasuhara T, Maki M, et al. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol. 2008;85:318–34. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- [8].Hess DC, Borlongan CV. Cell-based therapy in ischemic stroke. Expert Rev Neurother. 2008;8:1193–201. doi: 10.1586/14737175.8.8.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hess DC, Borlongan CV. Stem cells and neurological diseases. Cell Prolif. 2008;1:94–114. doi: 10.1111/j.1365-2184.2008.00486.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lapidot T, Kollet O. The brain-bone-blood triad: traffic lights for stem-cell homing and mobilization. Hematology Am Soc Hematol Educ Program. 2010;2010:1–6. doi: 10.1182/asheducation-2010.1.1. [DOI] [PubMed] [Google Scholar]
- [11].Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–10. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- [12].Nervi B, Link DC, DiPersio JF. Cytokines and hematopoietic stem cell mobilization. J Cell Biochem. 2006;99:690–70. doi: 10.1002/jcb.21043. [DOI] [PubMed] [Google Scholar]
- [13].Papayannopoulou T, Scadden DT. Stem-cell ecology and stem cells in motion. Blood. 2008;111:3923–30. doi: 10.1182/blood-2007-08-078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dar A, Kollet O, Lapidot T. Mutual, reciprocal SDF-1/CXCR4 interactions between hematopoietic and bone marrow stromal cells regulate human stem cell migration and development in NOD/SCID chimeric mice. Exp Hematol. 2006;34:967–75. doi: 10.1016/j.exphem.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [15].Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Tissue Kinet. 1997;3:393–403. doi: 10.1111/j.1365-2184.1970.tb00347.x. [DOI] [PubMed] [Google Scholar]
- [16].Gronthos S, Simmons PJ. The biology and application of human bone marrow stromal cell precursors. J Hematother. 1996;5:15–23. doi: 10.1089/scd.1.1996.5.15. [DOI] [PubMed] [Google Scholar]
- [17].Haynesworth SE, Baber MA, Caplan AI. Cell surface antigens on human marrow-derived mesenchymal cells are detected by monoclonal antibodies. Bone. 1992;13:69–80. doi: 10.1016/8756-3282(92)90363-2. [DOI] [PubMed] [Google Scholar]
- [18].Muraglia A, Cancedda R, Quarto R. Clonal mesenchymal progenitors from human bone marrow differentiate in vitro according to a hierarchical model. J Cell Sci. 2000;113:1161–6. doi: 10.1242/jcs.113.7.1161. [DOI] [PubMed] [Google Scholar]
- [19].Pereira RF, O’Hara MD, Laptev AV, et al. Marrow stromal cells as a source of progenitor cells for nonhematopoietic tissues in transgenic mice with a phenotype of osteogenesis imperfecta. Proc Natl Acad Sci USA. 1999;95:1142–47. doi: 10.1073/pnas.95.3.1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- [21].Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann. Neurol. 2005;57:874–82. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- [22].Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- [23].Chen J, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001;32:1005–11. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- [24].Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- [25].Li Y, Chen J, Chopp M. Adult bone marrow transplantation after stroke in adult rats. Cell Transplant. 2001;10:31–40. [PubMed] [Google Scholar]
- [26].Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–23. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- [27].Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–17. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- [28].Rempe DA, Kent TA. Using bone marrow stromal cells for treatment of stroke. Neurology. 2002;59:486–7. doi: 10.1212/wnl.59.4.486. [DOI] [PubMed] [Google Scholar]
- [29].Song S, Kamath S, Mosquera D, et al. Expression of brain natriuretic peptide by human bone marrow stromal cells. Exp. Neurol. 2004;185:191–7. doi: 10.1016/j.expneurol.2003.09.003. [DOI] [PubMed] [Google Scholar]
- [30].Tang Y, Yasuhara T, Hara K, et al. Transplantation of Bone Marrow-Derived Stem Cells:A Promising Therapy for Stroke. Cell Transplant. 2007;16:159–69. [PubMed] [Google Scholar]
- [31].Kucia M, Reca R, Campbell FR, et al. A population of very small embryonic-like (VSEL) CXCR4 (+) SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–69. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- [32].Kucia M, Ratajczak J, Ratajczak MZ. Are bone marrow stem cells plastic or heterogenous - that is the question. Exp Hematol. 2005;33:613–23. doi: 10.1016/j.exphem.2005.01.016. [DOI] [PubMed] [Google Scholar]
- [33].Zuba-Surma EK, Kucia M, Wu W, et al. Very small embryonic-like stem cells are present in adult murine organs: Image Stream-based morphological analysis and distribution studies. Cytometry. A. 2008;73A:1116–27. doi: 10.1002/cyto.a.20667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kucia M, Halasa M, Wysoczynski M, et al. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood: preliminary report. Leukemia. 2007;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- [35].Asahara T, Murohara T, Sullivan A, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- [36].Asahara T, Masuda H, Takahashi T, et al. Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res. 1999;85:221–8. doi: 10.1161/01.res.85.3.221. [DOI] [PubMed] [Google Scholar]
- [37].Heissig B, Hattori K, Dias S, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–37. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Lin Y, Weisdorf DJ, Solovey A, Hebbel RP. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–7. doi: 10.1172/JCI8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].McCarty JH. Cell adhesion and signaling networks in brain neurovascular units. Curr Opin Hematol. 2009;16:209–14. doi: 10.1097/MOH.0b013e32832a07eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Griese DP, Ehsan A, Melo LG, et al. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: Implications for cell- based vascular therapy. Circulation. 2003;108:2710–15. doi: 10.1161/01.CIR.0000096490.16596.A6. [DOI] [PubMed] [Google Scholar]
- [41].Murohara T, Ikeda H, Duan J, et al. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J Clin Invest. 2000;105:1527–36. doi: 10.1172/JCI8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lapergue B, Mohammad A, Shuaib A. Endothelial progenitor cells and cerebroascular diseases. Prog Neurobiol. 2007;83:349–62. doi: 10.1016/j.pneurobio.2007.08.001. [DOI] [PubMed] [Google Scholar]
- [43].Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34(+) cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–58. [PubMed] [Google Scholar]
- [44].Rouhl RP, van Oostenbrugge RJ, Damoiseaux J, Tervaert JW, Lodder J. Endothelial progenitor cell research in stroke: a potential shift in pathophysiological and therapeutical concepts. Stroke. 2008;39:2158–65. doi: 10.1161/STROKEAHA.107.507251. [DOI] [PubMed] [Google Scholar]
- [45].Nishimura N, Schaffer CB, Friedman B, Tsai PS, Lyden PD, Klein-feld D. Targeted insult to subsurface cortical blood vessels using ultrashort laser pulses: three models of stroke. Nat Methods. 2006;3:99–108. doi: 10.1038/nmeth844. [DOI] [PubMed] [Google Scholar]
- [46].Rivers CS, Wardlaw JM, Armitage PA, et al. Persistent infarct hyperintensity on diffusion- weighted imaging late after stroke indicates heterogeneous, delayed, infarct evolution. Stroke. 2006;37:1418–23. doi: 10.1161/01.STR.0000221294.90068.c4. [DOI] [PubMed] [Google Scholar]
- [47].Ay H, Arsava EM, Rosand J, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–13. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- [48].Stoeckel MC, Wittsack HJ, Meisel S, Seitz RJ. Pattern of cortex and white matter involvement in severe middle cerebral artery ischemia. J Neuroimaging. 2007;17:131–40. doi: 10.1111/j.1552-6569.2007.00102.x. [DOI] [PubMed] [Google Scholar]
- [49].Hristov M, Weber C. Endothelial progenitor cells: characterization, pathophysiology, and possible clinical relevance. J Cell Mol Med. 2004;8:498–508. doi: 10.1111/j.1582-4934.2004.tb00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Taguchi A, Matsuyama T, Moriwaki H, et al. Circulating cd34-positive cells provide an index of cerebrovascular function. Circulation. 2004;109:2972–75. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]
- [51].Gehling UM, Ergün S, Schumacher U, et al. In vitro differentiation of endothelial cells from AC133-positive progenitor cells. Blood. 2000;95:3106–12. [PubMed] [Google Scholar]
- [52].Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–31. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- [53].Rustemeyer P, Wittkowski W, Jurk K, Koller A. Optimized flow cytometric analysis of endothelial progenitor cells in peripheral blood. J Immunoassay Immunochem. 2006;27:77–88. doi: 10.1080/15321810500403789. [DOI] [PubMed] [Google Scholar]
- [54].Takahashi T, Kalka C, Masuda H, et al. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–8. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- [55].Schatteman GC, Awad O. Hemangioblasts, angioblasts, and adult endothelial cell progenitors. Anat Rec A Discov Mol Cell Evol Biol. 2004;276:13–21. doi: 10.1002/ar.a.10131. [DOI] [PubMed] [Google Scholar]
- [56].Khakoo AY, Finkel T. Endothelial progenitor cells. Annu Rev Med. 2005;56:79–101. doi: 10.1146/annurev.med.56.090203.104149. [DOI] [PubMed] [Google Scholar]
- [57].Kocher AA, Schuster MD, Bonaros N, et al. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol. 2006;40:455–64. doi: 10.1016/j.yjmcc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- [58].Cines DB, Pollak ES, Buck CA, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–61. [PubMed] [Google Scholar]
- [59].Ghani U, Shuaib A, Salam A, et al. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–3. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- [60].Hill JM, Zalos G, Halcox JP, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- [61].Borlongan CV, Skinner SJ, Geaney M, Vasconcellos AV, Elliott RB, Emerich DF. Intracerebral transplantation of porcine choroid plexus provides structural and functional neuroprotection in a rodent model of stroke. Stroke. 2004;35:2206–10. doi: 10.1161/01.STR.0000138954.25825.0b. [DOI] [PubMed] [Google Scholar]
- [62].Borlongan CV, Hadman M, Sanberg CD, Sanberg PR. Central nervous system entry of peripherally injected umbilical cord blood cells is not required for neuroprotection in stroke. Stroke. 2004;35:2385–89. doi: 10.1161/01.STR.0000141680.49960.d7. [DOI] [PubMed] [Google Scholar]
- [63].Bliss TM, Kelly S, Shah AK, et al. Transplantation of hNT neurons into the ischemic cortex: cell survival and effect on sensorimotor behavior. J Neurosci Res. 2006;83:1004–14. doi: 10.1002/jnr.20800. [DOI] [PubMed] [Google Scholar]
- [64].Chen J, Cui X, Zacharek A, et al. Niaspan increases angiogenesis and improves functional recovery after stroke. Ann Neurol. 2007;62:49–58. doi: 10.1002/ana.21160. [DOI] [PubMed] [Google Scholar]
- [65].Manaenko A, Chen H, Kammer J, Zhang JH, Tang J. Comparison Evans Blue injection routes: Intravenous versus intraperitoneal, for measurement of blood-brain barrier in a mice hemorrhage model. J Neurosci Methods. 2011;195:206–10. doi: 10.1016/j.jneumeth.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Chopp M, Zhang ZG, Jiang Q. Neurogenesis, angiogenesis, and MRI indices of functional recovery from stroke. Stroke. 2007;38:827–31. doi: 10.1161/01.STR.0000250235.80253.e9. [DOI] [PubMed] [Google Scholar]
- [67].Guzman R, Uchida N, Bliss TM, et al. Long-term monitoring of transplanted human neural stem cells in developmental and pathological contexts with MRI. Proc Natl Acad Sci USA. 2007;104:10211–6. doi: 10.1073/pnas.0608519104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Borlongan CV. Bone marrow stem cell mobilization in stroke: a ‘bonehead’ may be good after all! Leukemia. 2011;25(11):1674–86. doi: 10.1038/leu.2011.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Borlongan CV, Glover LE, Tajiri N, Kaneko Y, Freeman TB. The great migration of bone marrow-derived stem cells toward the ischemic brain: Therapeutic implications for stroke and other neurological disorders. Prog Neurobiology. 2011;95(2):213–28. doi: 10.1016/j.pneurobio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]