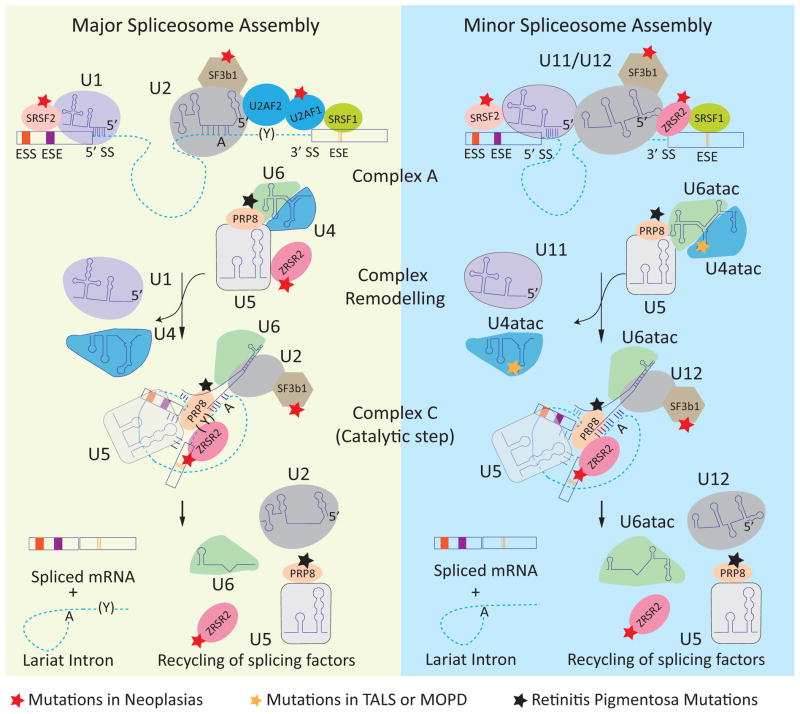
Figure 1.
Spliceosome assembly and disease-associated mutations in spliceosome components. The dashed lines and rectangles represent introns and exons, respectively. The left panel shows the assembly of the major (U2 type) spliceosome. U1 and U2 snRNPs are recruited to the consensus 5′ splice site (5′ SS) and branch point (A), respectively. The U2-auxiliary factor heterodimer (U2AF2/U2AF1) interacts with the polypyrimidine track (Y) and 3′ splice site (3′ SS), forming complex A. The U4/6 and U5 snRNPs join the assembling spliceosome followed by remodeling of the complex leading to removal of the U1 and U4 snRNP and formation of the catalytic complex (complex C). Two trans-esterification reactions join the exons and release an intron lariat that is subsequently degraded and the spliceosome components are recycled for subsequent rounds of splicing. The right panel shows the assembly of the minor (U12 type) spliceosome, in which U1, U2, U4, and U6 are replaced by homologous U11, U12, U4atac, and U6atac snRNPs, respectively. The red star indicates the components that are mutated in neoplasias. The black star indicates the components that are mutated in retinitis pigmentosa. The orange star indicates the mutation in U4atac that is associated with MOPD1. Abbreviations: ESE, exonic splicing enhancers; ESS, exonic splicing silencers.