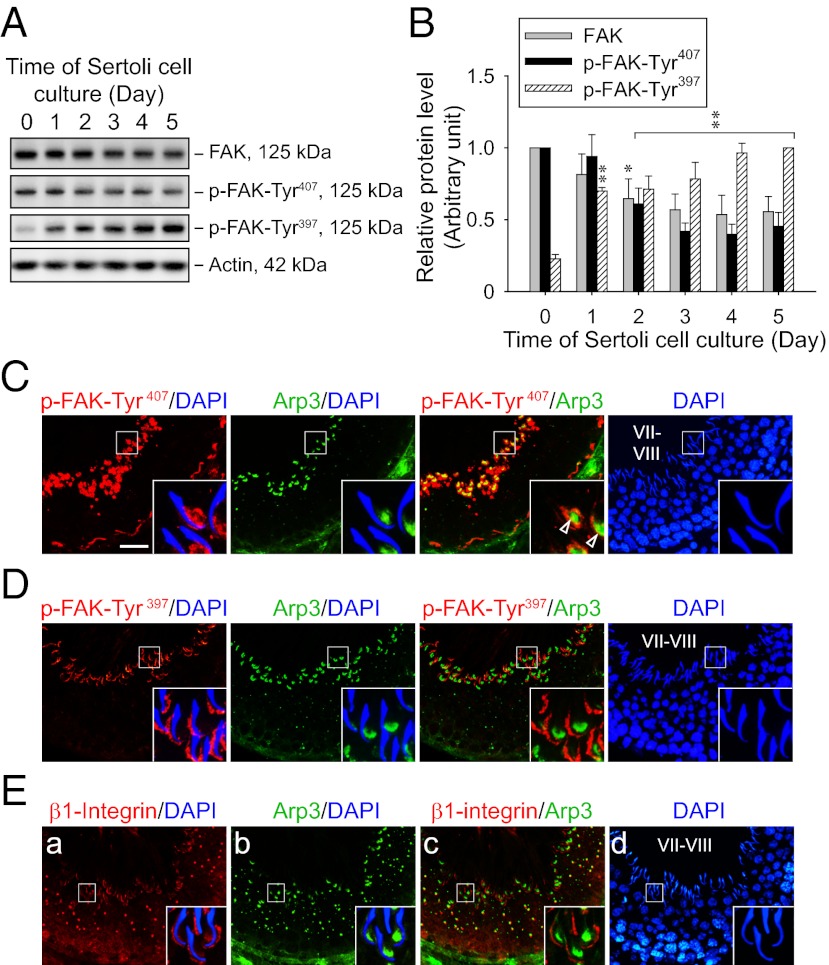
Fig. 2.
FAK phosphorylated at Tyr407 and Tyr397 shows restricted but differential temporal and spatial expression in the seminiferous epithelium of adult rat testes. (A) Immunoblots show the change in protein levels of FAK and its phosphorylated forms in Sertoli cells (0.4 × 106 cells/cm2) cultured for specified durations after their isolation. Lysate containing 25 μg of proteins was loaded in each lane. Actin served as a control for subsequent quantification analysis. (B) Histogram summarizes immunoblotting results as in A from several independent experiments. Each data point was normalized against the corresponding actin level, and the protein level was arbitrarily set at 1 either on day 0 for FAK and p-FAK-Tyr407 or on day 5 for p-FAK-Tyr397. Each bar represents a mean ± SD of n = 3–4. *P < 0.05; **P < 0.01 vs. day 0; one-way ANOVA followed by Dunnett’s test. Dual-labeled immunofluorescence staining of p-FAK-Tyr407 (C; red), p-FAK-Tyr397 (D; red), or β1-integrin (E; red) with Arp3 (C–E; green) in frozen sections of adult rat testes. Nuclei were visualized with DAPI (blue). (Insets) Boxed area in each image in C–E was magnified. In stage VII–VIII tubules, both p-FAK-Tyr407 and Arp3 were predominantly localized at the concave side of elongated spermatid heads, partially colocalizing with each other (open arrowheads in C), whereas p-FAK-Tyr397 and β1-integrin were mainly found at the convex side instead. (Scale bar: C–E, 40 μm.)