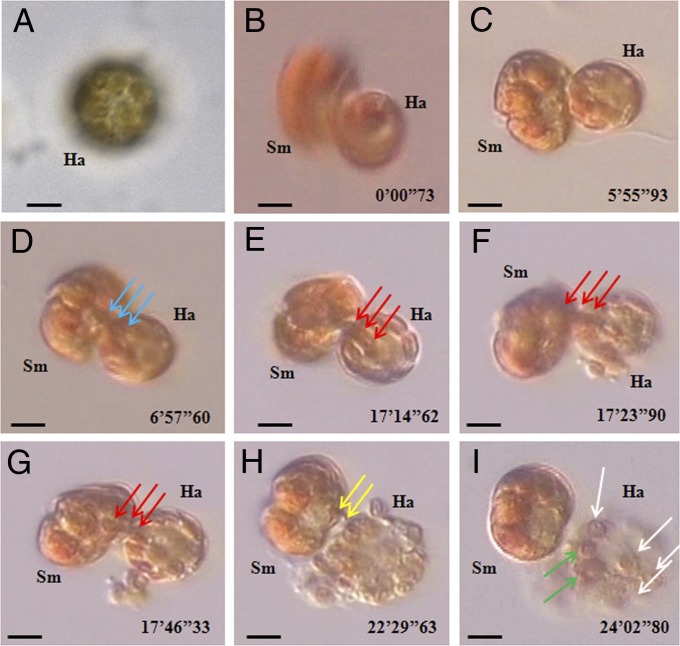
Fig. 3.
Feeding processes of free-living Symbiodinium cells on H. akashiwo prey, observed under an inverted microscope and recorded by video microscopy. (A) An unfed H. akashiwo (Ha) cell containing many chloroplasts (20–35 chloroplasts per cell). A Symbiodinium cell (Sm) feeding on an H. akashiwo (Ha) prey after deploying a tow filament and then a peduncle (stage 1), as shown in Fig. 1 K–M. (B–D) A Symbiodinium cell (Sm) feeding on an H. akashiwo (Ha) prey with spinning (stage 2). In stage 2, several prey chloroplasts were sucked by the predator. (E–G) A Symbiodinium cell (Sm) feeding on an H. akashiwo prey (Ha) after it had stopped rotating (stage 3). In stage 3, additional prey chloroplasts (blue and red arrows indicate different chloroplasts) were sucked by the predator again, after which the prey chloroplasts collapsed and burst, most likely owing to large empty spaces where prey chloroplasts occurred previously. Approximately 8–10 chloroplasts were observed within the burst prey cell. (H and I) Additional prey chloroplasts (yellow arrows) were sucked again by the predator from the burst prey cell (stage 4). At the end of stage 4, several empty (i.e., completely digested, white arrows) and one or two thylakoid-retained (green arrows) chloroplasts were observed inside the prey cell (I). (Scale bars: 5 μm.)