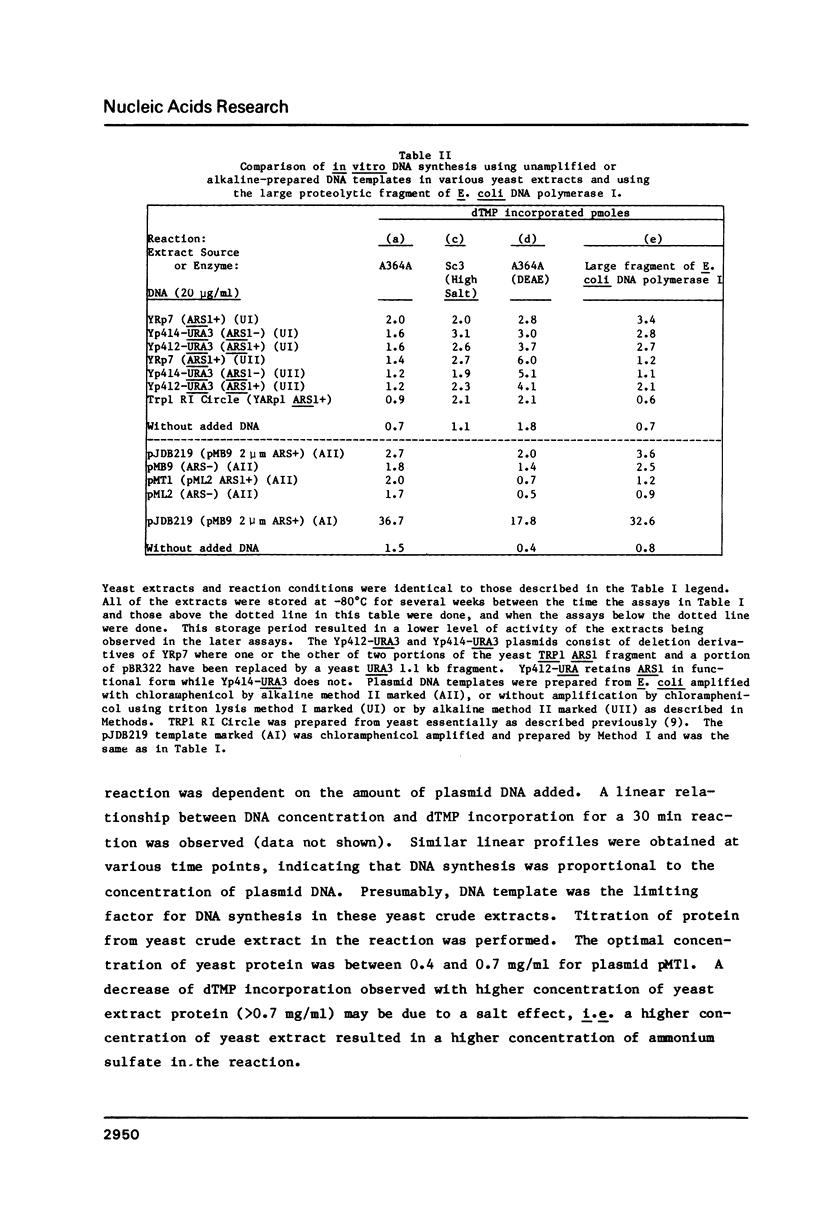
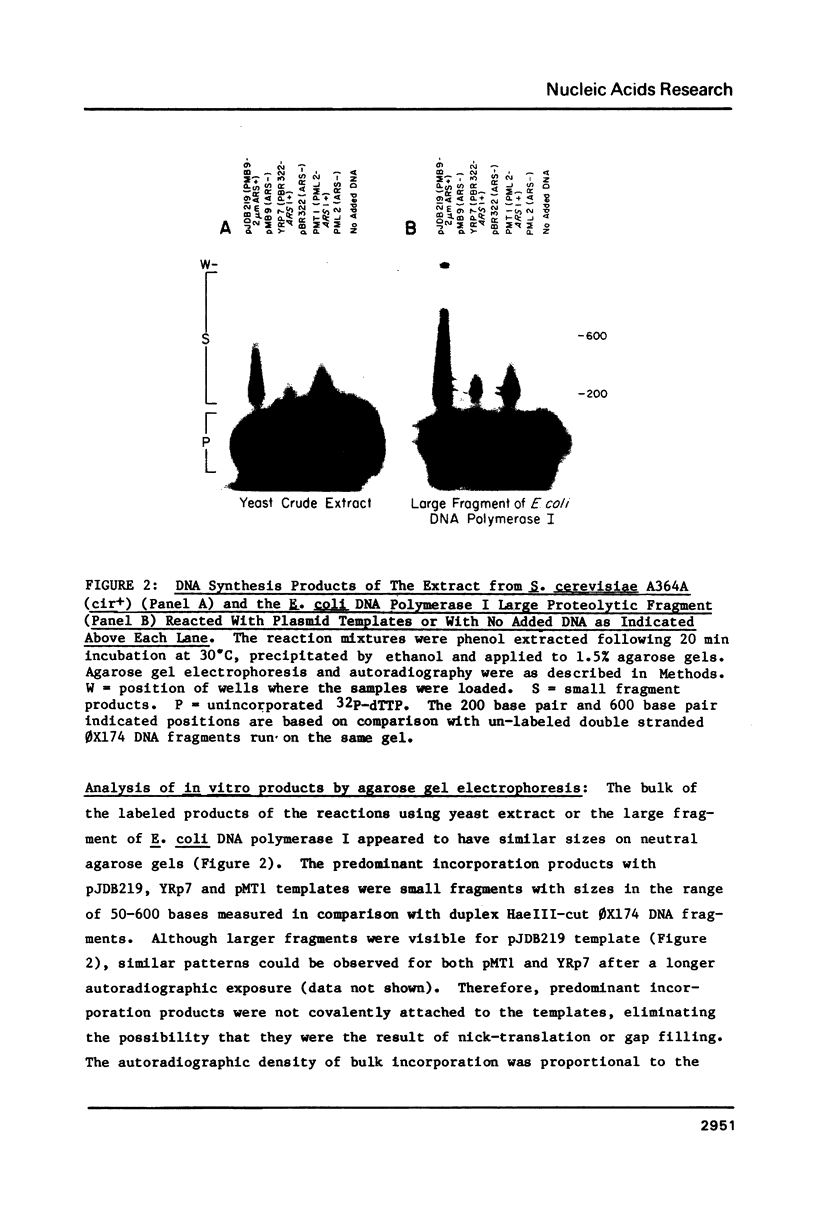
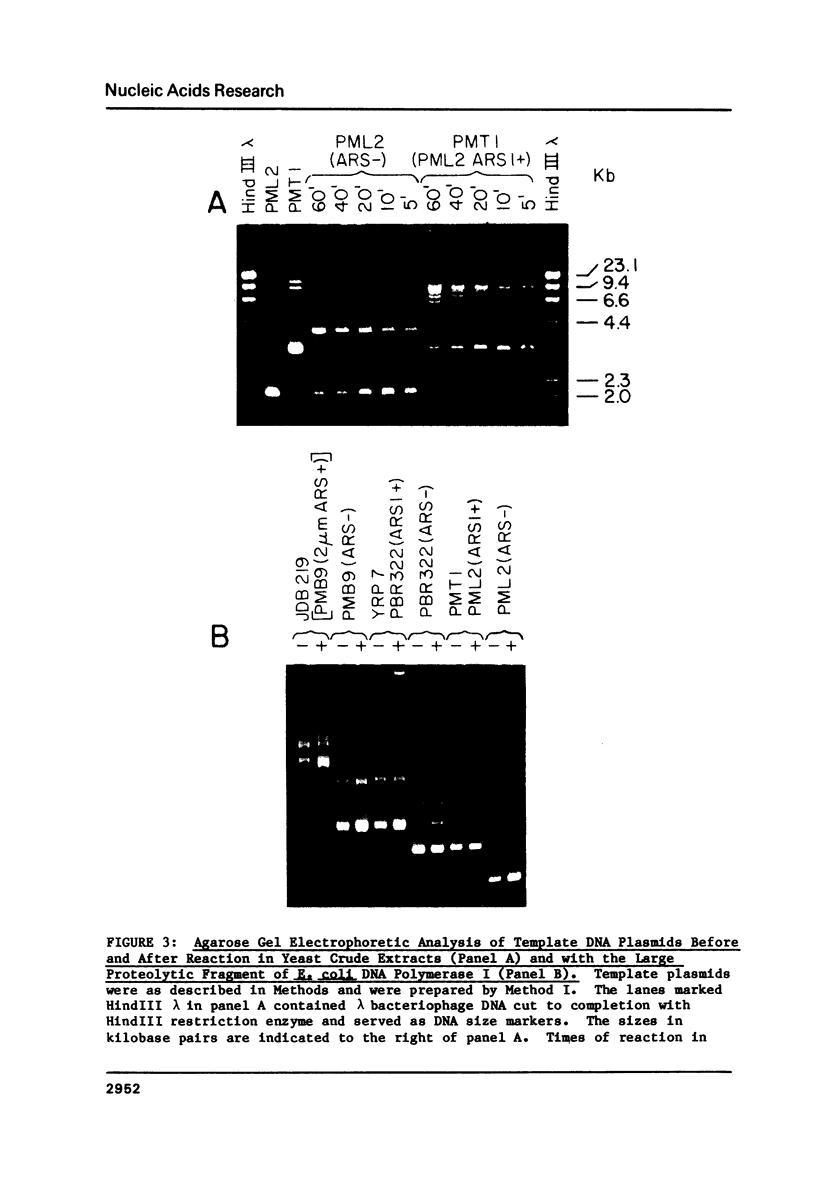
Abstract
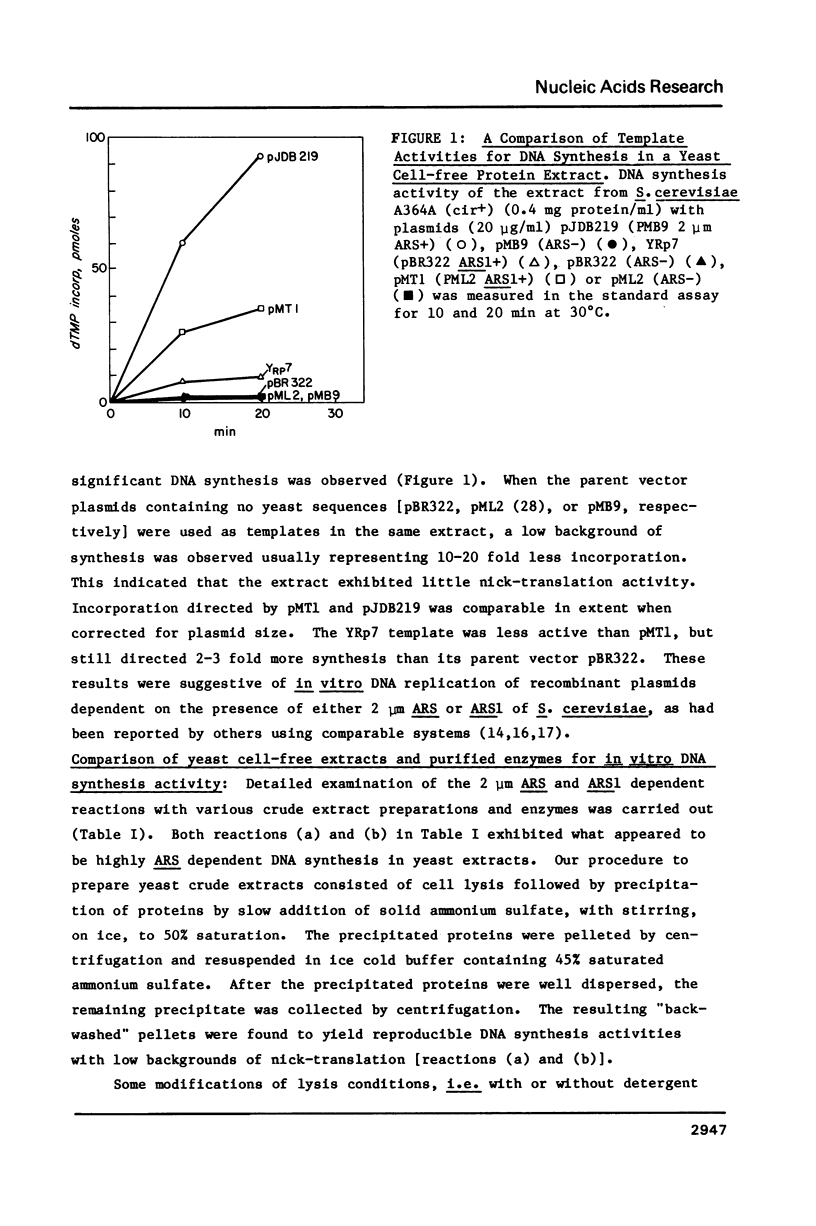
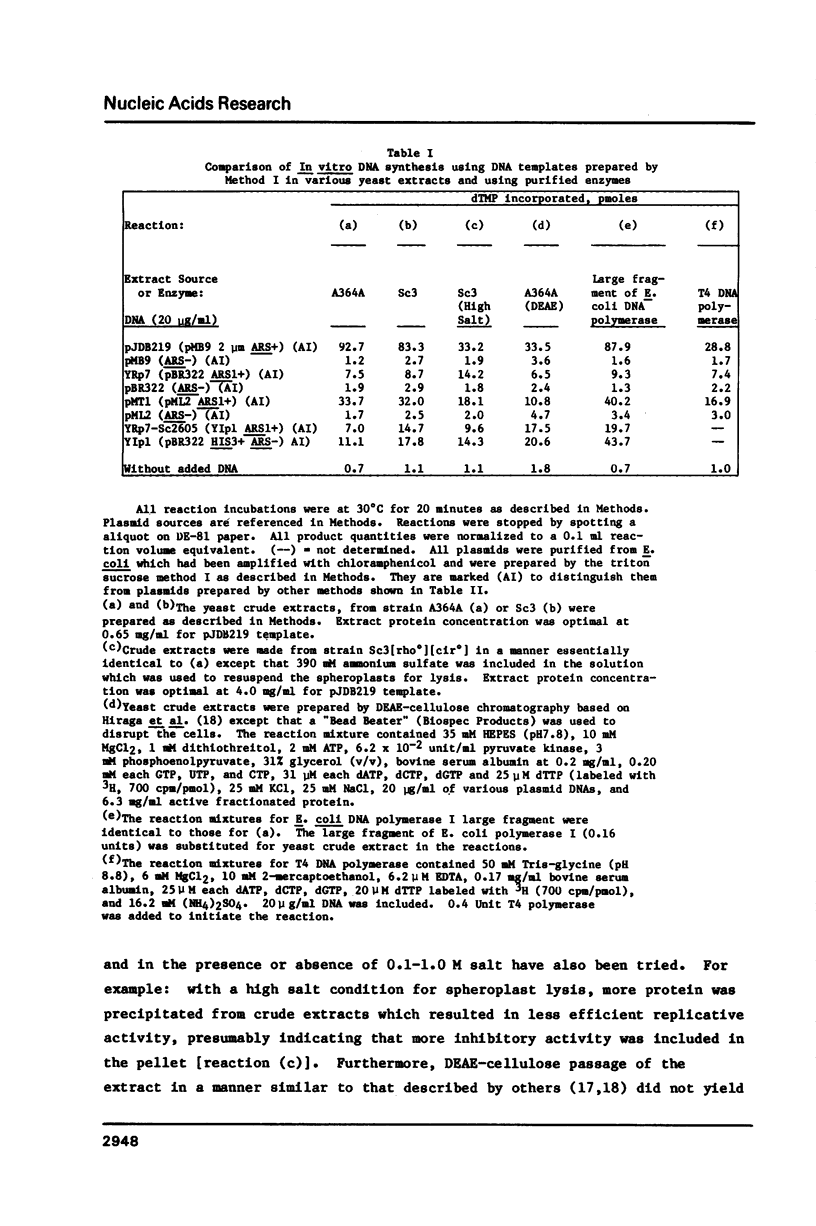
In our attempts to establish a cell-free DNA replication system for the yeast Saccharomyces cerevisiae, we have observed that recombinant DNA plasmids purified from Escherichia coli by a common procedure (lysozyme-detergent lysis and equilibrium banding in cesium chloride ethidium bromide gradients) often serve as templates for DNA synthesis by elongation enzymes. The templates could be elongated equally well by enzymes present in the yeast cell-free extracts, by the large proteolytic fragment of E. coli DNA polymerase I or by T4 DNA polymerase. The template activity of the purified plasmids was dependent on the presence of heterologous DNA segments in the bacterial vectors. The template activity could be diminished by treatment with alkali. We propose that the ability of recombinant plasmids isolated from bacterial hosts to serve as elongation templates may lead to erroneous conclusions when these plasmids are used as templates for in vitro replication or transcription reactions.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beggs J. D. Transformation of yeast by a replicating hybrid plasmid. Nature. 1978 Sep 14;275(5676):104–109. doi: 10.1038/275104a0. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Betlach M. C., Boyer H. W. Construction and characterization of new cloning vehicles. I. Ampicillin-resistant derivatives of the plasmid pMB9. Gene. 1977;2(2):75–93. doi: 10.1016/0378-1119(77)90074-9. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Broach J. R. The yeast plasmid 2 mu circle. Cell. 1982 Feb;28(2):203–204. doi: 10.1016/0092-8674(82)90337-3. [DOI] [PubMed] [Google Scholar]
- Celniker S. E., Campbell J. L. Yeast DNA replication in vitro: initiation and elongation events mimic in vivo processes. Cell. 1982 Nov;31(1):201–213. doi: 10.1016/0092-8674(82)90420-2. [DOI] [PubMed] [Google Scholar]
- Chan C. S., Tye B. K. Autonomously replicating sequences in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6329–6333. doi: 10.1073/pnas.77.11.6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fangman W. L., Hice R. H., Chlebowicz-Sledziewska E. ARS replication during the yeast S phase. Cell. 1983 Mar;32(3):831–838. doi: 10.1016/0092-8674(83)90069-7. [DOI] [PubMed] [Google Scholar]
- Hartley J. L., Donelson J. E. Nucleotide sequence of the yeast plasmid. Nature. 1980 Aug 28;286(5776):860–865. doi: 10.1038/286860a0. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Sudo T., Yoshida M., Kubota H., Ueyama H. In vitro replication of recombinant plasmids carrying chromosomal segments of Xenopus laevis. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3697–3701. doi: 10.1073/pnas.79.12.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao C. L., Carbon J. High-frequency transformation of yeast by plasmids containing the cloned yeast ARG4 gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3829–3833. doi: 10.1073/pnas.76.8.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman B. C., Cramer J. H., Rownd R. H. Properties of a Saccharomyces cerevisiae mtDNA segment conferring high-frequency yeast transformation. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1578–1582. doi: 10.1073/pnas.79.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Edelman G. M. Replication in vitro of the 2-micrometer DNA plasmid of yeast. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1223–1227. doi: 10.1073/pnas.76.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo H., Greenberg B. D., Sugino A. Yeast 2-micrometer plasmid DNA replication in vitro: origin and direction. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7261–7265. doi: 10.1073/pnas.78.12.7261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavialle C., Sekura R., Madden M. J., Salzman N. P. Interaction between calf thymus RNA polymerase II and singly nicked Simian virus 40 DNA. J Biol Chem. 1982 Oct 25;257(20):12458–12466. [PubMed] [Google Scholar]
- Lusky M., Botchan M. Inhibition of SV40 replication in simian cells by specific pBR322 DNA sequences. Nature. 1981 Sep 3;293(5827):79–81. doi: 10.1038/293079a0. [DOI] [PubMed] [Google Scholar]
- Petes T. D. Molecular genetics of yeast. Annu Rev Biochem. 1980;49:845–876. doi: 10.1146/annurev.bi.49.070180.004213. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheinin R., Humbert J. Some aspects of eukaryotic DNA replication. Annu Rev Biochem. 1978;47:277–316. doi: 10.1146/annurev.bi.47.070178.001425. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Thomas M., Kelly J., Selker E., Davis R. W. Eukaryotic DNA segments capable of autonomous replication in yeast. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4559–4563. doi: 10.1073/pnas.77.8.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Scherer S., Davis R. W. High-frequency transformation of yeast: autonomous replication of hybrid DNA molecules. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1035–1039. doi: 10.1073/pnas.76.3.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanofsky C., Horn V., Bonner M., Stasiowski S. Polarity and enzyme functions in mutants of the first three genes of the tryptophan operon of Escherichia coli. Genetics. 1971 Dec;69(4):409–433. doi: 10.1093/genetics/69.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarbrough L. R. Utilization of primers and primer-templates by wheat germ RNA polymerase II. J Biol Chem. 1982 Jun 10;257(11):6171–6177. [PubMed] [Google Scholar]
- Zakian V. A., Brewer B. J., Fangman W. L. Replication of each copy of the yeast 2 micron DNA plasmid occurs during the S phase. Cell. 1979 Aug;17(4):923–934. doi: 10.1016/0092-8674(79)90332-5. [DOI] [PubMed] [Google Scholar]
- Zakian V. A. Origin of replication from Xenopus laevis mitochondrial DNA promotes high-frequency transformation of yeast. Proc Natl Acad Sci U S A. 1981 May;78(5):3128–3132. doi: 10.1073/pnas.78.5.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakian V. A., Scott J. F. Construction, replication, and chromatin structure of TRP1 RI circle, a multiple-copy synthetic plasmid derived from Saccharomyces cerevisiae chromosomal DNA. Mol Cell Biol. 1982 Mar;2(3):221–232. doi: 10.1128/mcb.2.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]