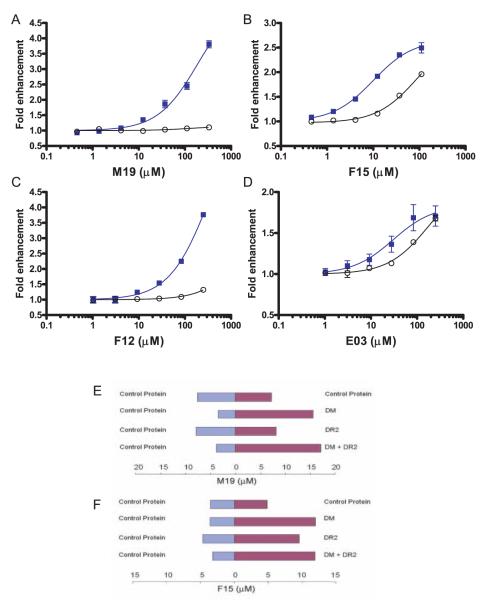
FIGURE 2.
Targets of the small molecules that accelerate peptide exchange. A–D, The kinetics of the peptide binding reaction were measured over a wide range of small molecule concentrations, both in the presence and absence of DM (■ and ○, respectively). The initial rate of the reactions was determined, and the fold enhancement over reactions without small molecules was plotted as a function of small molecule concentration. Such graphs are shown in A–D for each of the small molecules (M19, F15, F12, and E03). The M19 and F12 enhancers (A and C) only accelerated the peptide binding reaction in the presence of DM. For the F15 and E03 enhancers (B and D), an acceleration of the peptide binding reaction was also observed in the absence of DM, but these molecules were most effective in the presence of DM. E–F, Binding of small molecules was examined in microdialysis experiments in which two compartments carrying a control protein (aldolase, blue bars) or an experimental protein (red bars) were separated by a dialysis membrane. Following overnight incubation at 4°C, the concentration of the small molecule in both compartments was measured. E, M19 was found to bind to DM but not to DR2 because it was only enriched when DM was present in the experimental compartment. F, The F15 enhancer was found to bind to both DM and DR2, consistent with the functional data shown in B. Microdialysis experiments were conducted three times with similar results, and a representative experiment in each case is shown.