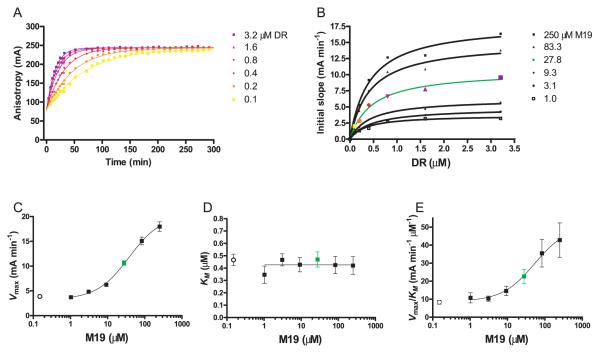
FIGURE 5.
Calculation of Vmax and KM values for DM in the presence and absence of the M19 small molecule. A, Peptide binding reactions were set up with a grid of six different substrate concentrations (DR2/CLIP, 0.1–3.2 μM; 30 nM MBP) and six concentrations of the M19 enhancer (1–250 μM). Shown is a set of six reactions with a fixed M19 concentration (27.8 μM) and six different DR2/CLIP concentrations. The initial slopes were calculated from anisotropy instead of FP for reasons described in Materials and Methods. B, For each concentration of M19, the initial slope of the reaction was plotted relative to the DR2/CLIP concentration. Each curve from A yielded one data point in B. From each of these curves, the maximum rate of the reaction (Vmax) and the substrate concentration (DR2/CLIP) required for half-maximal activity (KM) was calculated. C–E, The Vmax (C), KM (D), and Vmax/KM (E) values were then plotted as a function of the M19 concentration. The ○ in both graphs show Vmax (C), KM (D), and Vmax/KM (E) values in the absence of the small molecule.