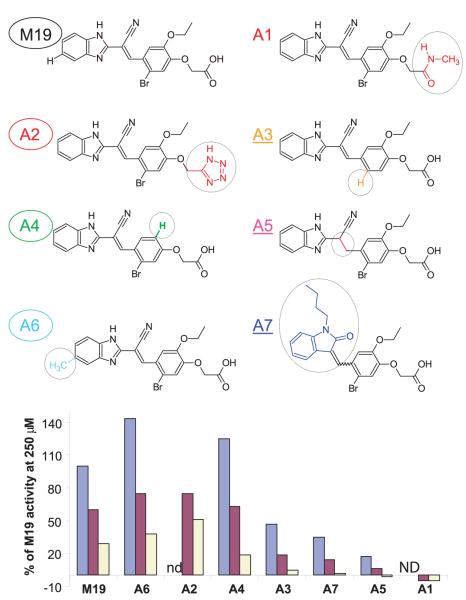
FIGURE 6.
Structural features of M19 that are important for activity. A series of M19 analogues with changes at defined sites (circled in each of the analogues) were tested at three concentrations (250, 50, and 10 μM); analogues A1 and A2 had limited solubility and were therefore tested only at 50 and 10 μM, but not at 250 μM (ND). Activity is expressed as percentage relative to M19 at a concentration of 250 μM. Analogues that have activity similar to or higher than M19 are circled (A2, A4, and A6), and analogues with reduced activity are underlined (A3, A5, and A7). The carboxylate was critical for activity because replacement with an amide (A1) resulted in a complete loss of activity, whereas substitution with a tetrazole (a carboxylic acid bioisostere) did not (A2). The ethyl-ether was not required, as shown by analog A4, and removal of the aryl bromide (A3) reduced but did not abrogate activity. Significant changes to the left portion of the molecule could be made as shown by analog A7, and addition of a methyl group to the benzimidazole ring (A6) yielded a compound with activity higher than M19.