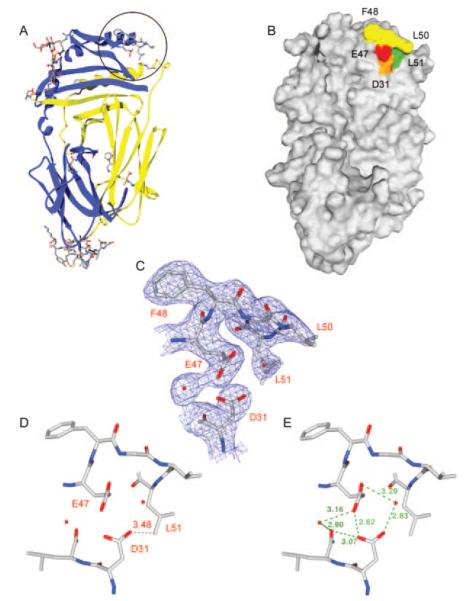
FIGURE 7.
Structural analysis of DM resolves the electron density for a hydrophobic ridge located in the DM β1 domain. A, A number of DM residues that were absent in PDB file 1HDM could be resolved in a higher resolution crystal structure and are shown as a stick representation on a ribbon diagram of DM. Five amino acids (DMβ 47–51, EFGLL) that form a hydrophobic ridge are circled. B, Molecular surface representation of DM in the same orientation as in A. Residues F48, G49, and L50 of the hydrophobic ridge are colored yellow, and residues βL51 and βE47 are colored green and red, respectively. A neighboring acidic residue (βD31) is colored orange. C, Electron density calculated from a 2Fobs-Fcalc map at 1σ shows that the DM β47–51 region is clearly resolved. Two neighboring water molecules and βD31 are also shown. The view is the same as in A and B. D–E, The distance between βD31 and βL51 as well as the hydrogen bonding network between the two acidic residues and the surrounding environment is shown. The view is tilted back slightly compared with A–C to clearly show the hydrogen bond distances.