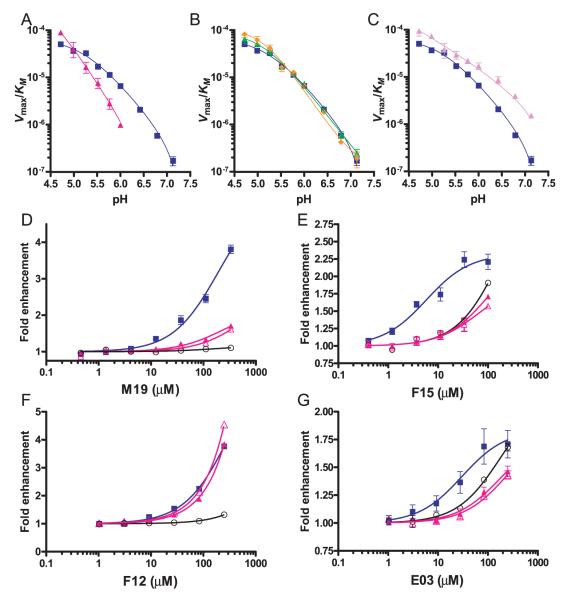
FIGURE 8.
Three of the four small molecule enhancers bind in the vicinity of the hydrophobic ridge of DM. A–C, Mutations were introduced at positions βL51, βE47, or βD31 of DMβ, and the activity of these DM mutants was examined as a function of pH in the absence of small molecule enhancers. Graphs show the activity of these mutants as Vmax/KM from pH 4.7 to 7.3. A, The βL51D mutation (red line) narrows the pH range of the enzyme relative to wildtype DM (blue). B–C, The individual βD31N (green) or βE47Q (orange) mutations (B) did not affect DM activity, whereas the βD31N-βE47Q double substitution (C, purple) increased the activity of the enzyme ~2-fold at a low pH (pH 4.7 and 5.0) and ~9-fold at a neutral pH (pH 7.3) compared with wild-type DM (blue). D–G, The effect of mutation of DM residue βL51 on the activity of the four small molecules. The dose response for each enhancer is shown in the presence of wild-type DM (blue line), the βL51D mutant (red, closed triangles), the βL51N mutant (red, open triangles), and in the absence of DM (black, open circles). Both βL51 mutations greatly reduced or abrogated the activity of M19 (D), F15 (E), and E03 (G).