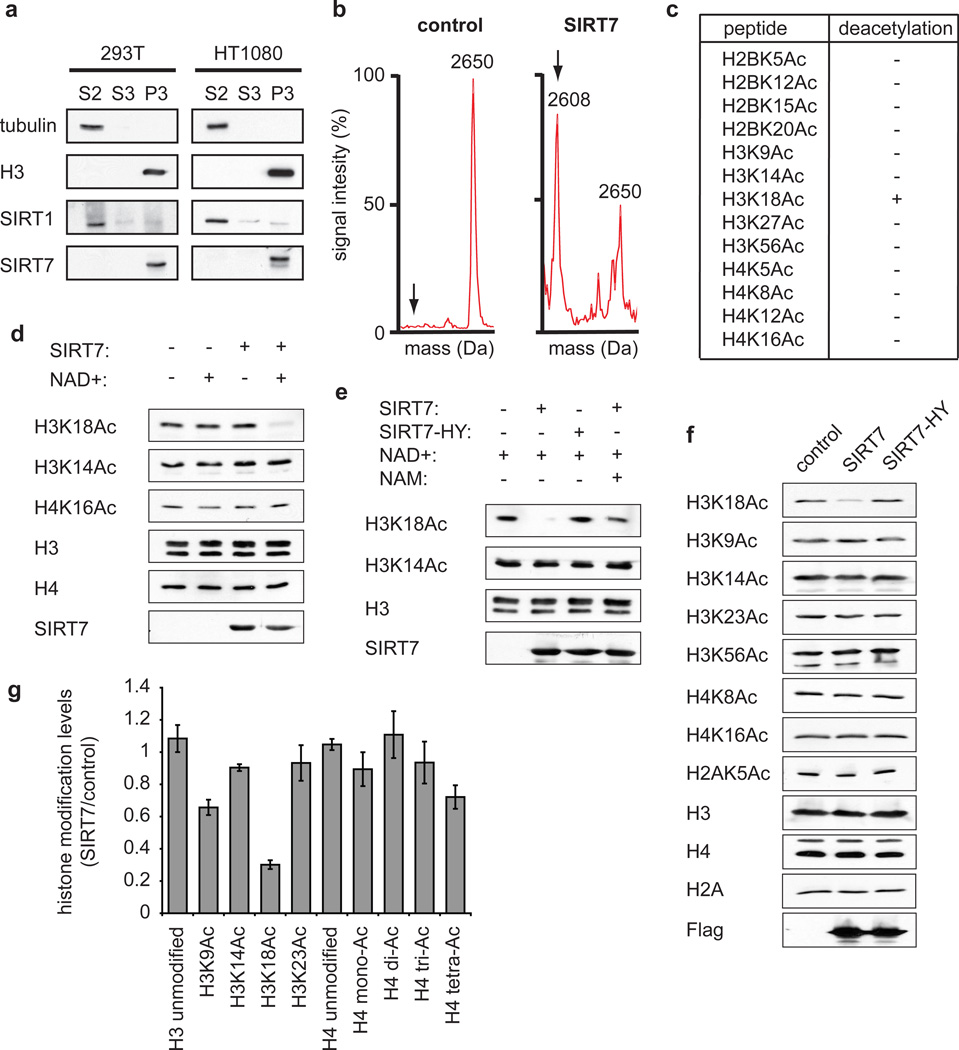
Figure 1. SIRT7 is a chromatin-associated H3K18Ac-specific deacetylase.
a, Western analysis showing chromatin association of SIRT7 in 293T and HT1080 cells. Biochemical fractions S2, S3, and P3 are enriched for cytoplasm, nucleoplasm, or chromatin, respectively. b, Mass spectra showing deacetylation of H3K18Ac peptide by SIRT7 compared to negative control reaction lacking enzyme. Molecular weights of acetylated and deacetylated (arrows) peptides are 2650 and 2608 Daltons, respectively. c, Results of SIRT7 deacetylation reactions using acetylated histone peptides, determined by mass spectrometry as in (b). d, e, Western analysis of H3K18Ac deacetylation activity of wild-type (SIRT7) or mutant (SIRT7-HY) proteins on poly-nucleosomes in vitro, and inhibition by nicotinamide (NAM). f, Western analysis showing H3K18Ac levels in 293T cells transfected with Flag-tagged SIRT7, SIRT7-HY, or control empty vector. g, Changes in global histone acetylation levels in SIRT7 overexpressing versus control 293T cells, determined by quantitative mass spectrometry. Error bars represent standard error of the mean (S.E.M.) of three independent experiments.