Figure 7.
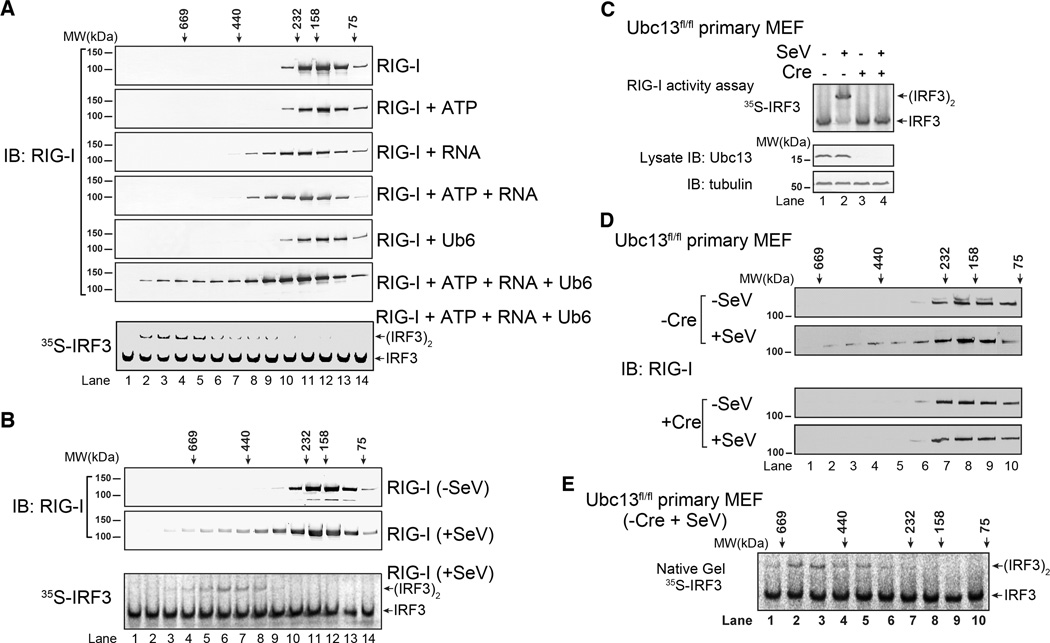
RNA and polyubiquitin chains induce oligomerization of full-length RIG-I. A. RIG-I was incubated with ATP, RNA and/or K63-Ub6 as indicated. The mixtures were fractionated using Superdex-200 gel filtration column. Aliquots of the fractions were analyzed by immunoblotting and IRF3 dimerization assay as indicated. Only RIG-I incubated with ATP, RNA and K63-Ub6 had IRF3 stimulatory activity (bottom, and data not shown). B. HEK293T cells stably expressing RIG-I-Flag were infected with SeV or uninfected. RIG-I-Flag was affinity purified, and analyzed by gel filtration using Superdex-200 column. Aliquots of the fractions were analyzed by immunoblotting and in vitro IRF3 dimerization assay. C. Ubc13fl/fl primary MEF cells were infected with a lentiviral vector expression RIG-I-Flag, then the Ubc13 gene was deleted with Cre recombinase or left intact (+ or − Cre). The cells were infected with SeV, and then RIG-I was affinity purified and tested for its ability to activate IRF3 dimerization (top). The efficiency of Ubc13 depletion was verified by immunoblotting (bottom). D. WT and Ubc13-deleted MEF cells (−/+ Cre) were infected with SeV or not infected, and then RIG-I was affinity purified and fractionated on a Superdex-200 column followed by immunoblotting. E. The fractions from RIG-I isolated from the virus-infected WT MEFs as shown in D (−Cre, + SeV) were analyzed for their ability to stimulate IRF3 dimerization. Results shown are representatives of two experiments.