Abstract
Dietary restriction (DR) extends lifespan and increases resistance to multiple forms of stress, including ischemia reperfusion injury to the brain and heart in rodents. While maximal effects on lifespan require long-term restriction, the kinetics of onset of benefits against acute stress are not known. Here we show that 2–4 weeks of 30% dietary restriction improved survival and kidney function following renal ischemia reperfusion injury in mice. Brief periods of water-only fasting were similarly effective at protecting against ischemic damage. Significant protection occurred within one day, persisted for several days beyond the fasting period and extended to another organ, the liver. Protection by both short-term DR and fasting correlated with improved insulin sensitivity, increased expression of markers of antioxidant defense and reduced expression of markers of inflammation and insulin/insulin-like growth factor-1 signaling. Unbiased transcriptional profiling of kidney from mice subject to short-term DR or fasting revealed a significant enrichment of signature genes of long-term DR. These data demonstrate that brief periods of reduced food intake, including short-term daily restriction and fasting, can increase resistance to ischemia reperfusion injury in rodents and suggest a rapid onset of benefits of DR in mammals.
Keywords: dietary restriction, ischemia reperfusion injury, fasting, oxidative stress, kidney, liver
Introduction
Dietary restriction (DR) encompasses a variety of interventions resulting in reduced nutrient and energy intake without malnutrition. DR is best known for its ability to extend lifespan in a wide variety of organisms (Weindruch et al. 1986; Masoro 2003; Bishop and Guarente 2007). Longevity effects were first reported in rodents in 1935 (McCay et al. 1935) and extended in subsequent decades to fish (Comfort 1963), worms (Klass 1977), flies (Partridge et al. 1987; Chippindale et al. 1993), yeast (Jiang et al. 2000; Lin et al. 2000) and non-human primates (Colman et al. 2009). Effects on human longevity are not known, but prospective studies show a favorable impact on markers of aging and predictors of long-term health, including improved cardiovascular fitness, body-mass index and insulin sensitivity (Heilbronn et al. 2006; Weiss et al. 2006; Fontana and Klein 2007).
The kinetics of onset and loss of longevity benefits of DR are best understood in the fruit fly Drosophila melanogaster. In young adult flies, maximal effects of DR on longevity, measured as a function of daily mortality rate, are achieved within 1–3 days of switching from a normal to a restricted diet and vice versa (Mair et al. 2003). The use of daily mortality rate as an endpoint in young adult rodents would require large numbers of animals due to low daily mortality rates and is thus considered impractical.
In addition to extended lifespan, another common property of organisms on DR is increased resistance to multiple forms of acute stress (Sinclair 2005; Brown-Borg 2006). In rodents, this includes resistance to paraquat toxicity and ischemia reperfusion injury. Paraquat is a free radical generator that primarily targets the lungs, and mice subject to ~5 or 8 months of 40% DR have a significant survival advantage over ad libitum fed animals (Sun et al. 2001; Richardson et al. 2004). Ischemia reperfusion injury is initiated by a lack of blood flow (ischemia) resulting in a state of tissue oxygen and nutrient deprivation characterized chiefly by ATP depletion, loss of ion gradients across membranes and buildup of toxic byproducts. Restoration of blood flow (reperfusion) causes further damage at first by inappropriate activation of cellular oxidases and subsequently by inflammatory mediators in response to tissue damage (Friedewald and Rabb 2004). Rodents on a restricted diet for 3 months to 1 year have reduced damage upon ischemic injury to the heart and brain in models of heart attack and stroke, respectively (Yu and Mattson 1999; Chandrasekar et al. 2001; Ahmet et al. 2005).
The length of time on a restricted diet required for the onset of increased stress resistance is not well characterized in any organism. Here, we examined the kinetics of onset and loss of protection against renal and hepatic ischemia reperfusion injury in mice using brief periods of food restriction, including 2–4 weeks of 30% reduced daily food availability (defined here as short-term DR) or 1–3 days of 100% restriction (water-only fasting).
Results
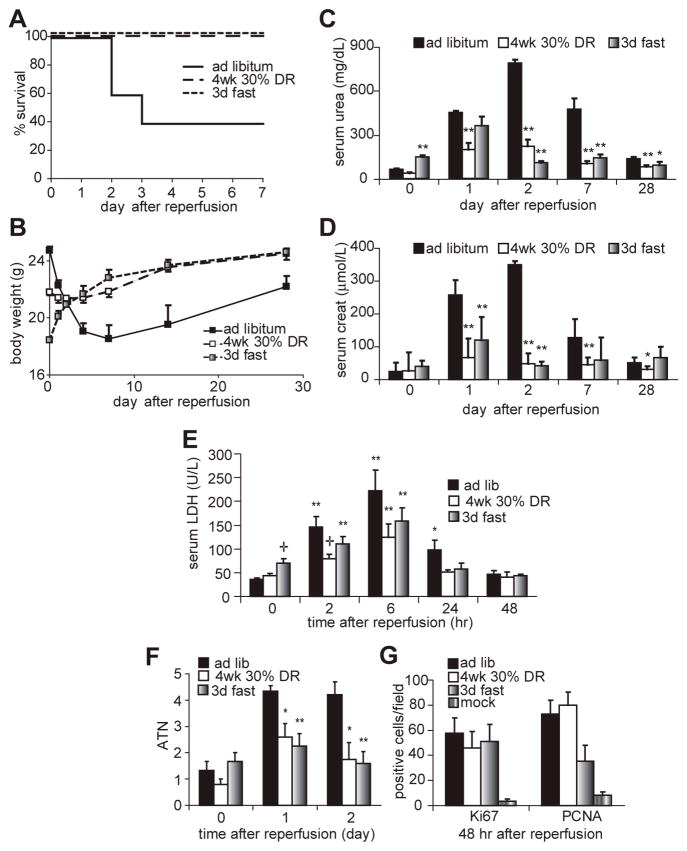
Bilateral renal ischemia was induced by clamping both renal pedicles for 37 minutes, followed by clamp removal to reinitiate blood flow. Under these conditions, 60% of control mice fed ad libitum prior to surgery died or were sacrificed due to morbidity (including excessive weight loss, drop in body temperature, ruffled fur, decreased activity and hunched body posture) and the associated buildup of toxic waste products (urea, creatinine) in the blood indicative of irreversible kidney failure by the fourth day following surgery (Fig 1A-D). In contrast, mice restricted daily to 70% of their ad libitum food intake (30% DR) for four weeks prior to challenge with renal IRI were protected from mortality, weight loss and kidney dysfunction (Fig 1A-D).
Figure 1. DR protects against the damaging effects of renal IRI.
A. Survival curves of male mice fed ad libitum, restricted to 70% of ad libitum food consumption for 4 weeks, or fasted for 3 days prior to induction of 37 minutes of bilateral renal IRI (n=10 per group). No further mortality was observed beyond day 4 after surgery. Both dietary treatments led to a significant survival advantage by Kaplan Meier analysis (log rank test, p<0.01).
B. Body weight of mice over a 28-day time course following renal IRI.
C, D. Kidney function as measured by serum urea (C) and creatinine (D) concentrations on the indicated days following surgery. Asterisks indicate the significance of the difference as compared to the ad libitum group at the same time point (* p<0.05; ** p<0.01).
E. Blood serum LDH of the indicated groups over a time course following reperfusion. Asterisks indicate significant differences relative to time 0 of the same treatment; crosses represent significant differences relative to the ad libitum group at the indicated time point.
F. Quantification of acute tubular necrosis on a 5 point scale before and after renal IRI based on blind scoring of hemotoxylin/eosin stained kidney sections as described previously (Leemans et al. 2005). Asterisks represent significant differences relative to the ad libitum group at the indicated time point (* p<0.05; ** p<0.01).
G. Percentage of cells expressing Ki67 or PCNA proliferative markers in a microscopic field of the indicated group on the second day following IRI. All groups were significantly elevated vs. the mock control.
To investigate shorter periods of more severe restriction, we tested the effects of water-only fasting. As with 4 wks of 30% DR, 3 days of fasting resulted in 100% survival, postoperative weight gain and reduced kidney dysfunction (Fig 1A-D). Protection afforded by both DR and fasting was further confirmed on the level of organ damage by longitudinal assessment of lactate dehydrogenase (LDH) in the serum (Fig 1E). Upon cellular damage or death, cytoplasmic LDH is released into the blood and can thus serve as a marker of acute tissue injury. Although LDH levels were significantly elevated in all groups 2 and 6 hours after reperfusion, they returned to preoperative levels 24 hours after reperfusion only in the DR and fasted groups. LDH levels were also significantly lower in the DR group at the 2 hour timepoint. Kidney damage was further assessed on the histological level by scoring acute tubular necrosis (ATN) on a five point scale (Leemans et al. 2005) before and after injury (Fig 1F). Both DR and fasted groups had significantly less ATN than the ad libitum fed group on days 1 and 2 after injury, consistent with better outcome. Following ischemic injury, damaged tubules have a limited capacity to regenerate. We measured cellular proliferation on the histological level using the proliferative markers PCNA and Ki67 (Fig 1G) on the second day following reperfusion coincident with the onset of organ regeneration. Both markers were elevated in animals subject to renal IRI relative to mock treated animals. There were no significant effects of dietary pretreatment on absolute proliferation levels. Taken together, these data are consistent with better outcome in the DR and fasted groups.
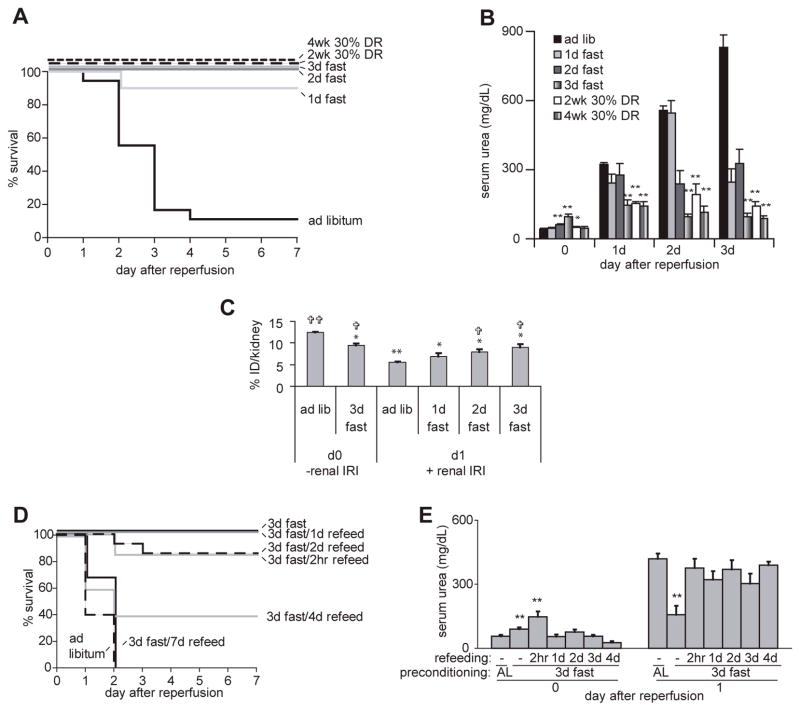
We next tested the kinetics of onset and loss of benefits of DR and fasting using a unilateral occlusion mode in which the left kidney was occluded for 37 minutes followed by removal of the right (undamaged) kidney. This model represents a more severe stress than the bilateral model with the same ischemic time because the recovery of renal function and animal survival depends on regeneration of a single damaged kidney. Under these conditions, 90% of ad libitum fed mice died or were sacrificed due to morbidity indicative of irreversible kidney failure by the fourth day following IRI. Mice on 30% DR for 4 weeks or 3 days had significantly elevated survival rates (both 100%; Fig 2A) and improved renal function (Fig 2B) as in the bilateral model. Two weeks of 30% DR was similarly effective as four weeks with respect to survival and kidney function (Fig 2A, B), indicating a rapid onset of benefits of DR. Similarly, 2 or even 1 day of water-only fasting prior to ischemia significantly elevated survival rates (100% and 90%, respectively, Fig 2A). Interestingly, despite maximal effects on survival within 2 days, protection against kidney dysfunction increased in a dose-dependent manner for up to 3 days, reaching protection levels similar to 2 or 4 weeks of 30% DR (Fig 2B).
Figure 2. Rapid onset and loss of protective effects of short-term DR and fasting.
A-C. Rapid onset: A. Survival curves of male mice fed ad libitum, 30% restricted for 2–4 weeks, or fasted for 1–3 days prior to induction of 37 minutes of unilateral renal IRI with contralateral nephrectomy (n=10–18 per group). B. Kidney function as measured by serum urea following 37 minutes of unilateral renal IRI in the indicated groups (serum from 4–10 individual animals was sampled per data point). Asterisks indicate the significance of the difference as compared to the ad libitum group at the same time point (** p<0.01). C. Mice fasted for 0–3 days (4–10 animals per group) were analyzed for kidney function either in the absence of renal ischemia (day 0, -renal IRI) or one day following 37 minutes renal ischemia (day 1, +renal IRI) by measuring radioactivity in the kidney with a gamma-counter 4 h after injection of 99mTc-DMSA, expressed as a percentage of the injected dose per one kidney (% ID/kidney). Note the reduced percentage of 99mTc-DMSA in the kidneys of the ad libitum group 24 hours after renal IRI (12.4% to 5.6%), indicative of renal dysfunction. The significance of the difference as compared to the ad libitum group prior to (asterisks) or one day after (crosses) renal IRI is indicated. D, E. Rapid loss: D. Survival curves of the indicated groups. Survival of animals refed for 2hr, 1 and 2 days was significantly different than ad libitum fed animals (p<0.002); survival of animals refed for 4 and 7 days was not significantly different than ad libitum fed animals. E. Kidney function as measured by serum urea prior to and one day following IRI. Data from three independent experiments with 4–12 animals per group are averaged. In the day 0 group asterisks indicate significant differences vs. the ad libitum fed control group; in the day 1 group, asterisks indicate significant difference between 3 days of fasting without refeeding and ad libitum (AL) fed animals as well as each of the refed groups (**p<0.01). There were no significant differences between the ad libitum group and any of the refed groups on day 1 following renal IRI.
Assessment of kidney function by serum-based measures including creatinine and urea can in some cases be affected by confounding factors such as differences in muscle mass or diet. We thus sought to confirm the rapid onset of protection afforded by fasting with a more direct measure of kidney function. The uptake of technetium-99m-radiolabeled dimercaptosuccinic acid (99mTc-DMSA) by the kidneys is directly related to kidney tubular reabsorption. 24 hours after IRI significantly less 99mTc-DMSA accumulated in the ad libitum group relative to the mock-treated animals, indicative of kidney dysfunction. In mice fasted 48 and 72 hours prior to IRI, this reduction was significantly ameliorated (Fig 2C).
In order to determine the kinetics of loss of the protective effect of fasting from renal IRI, we allowed animals ad libitum access to food for variable times following a three day fast and tested their resistance to ischemic injury. Significant benefits on animal survival remained for at least two days after refeeding but were lost after 4 days (Fig 2D). Despite these lingering survival benefits, protection from kidney dysfunction was lost by as little as two hours of refeeding prior to surgery (Fig 2E).
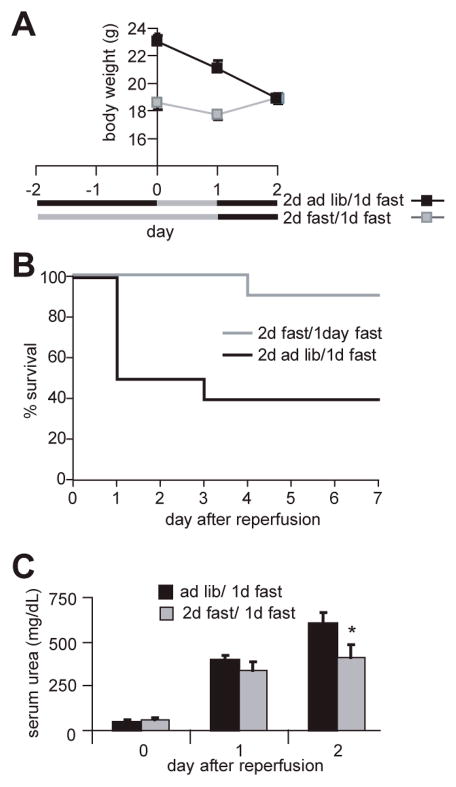
In contrast to ad libitum fed mice, food-restricted mice started eating shortly after awaking from surgery. As a result they gained weight rapidly following surgery, whereas the ad libitum fed mice did not eat in the first days following surgery and as a result lost weight (Fig 1B). We thus asked if benefits of fasting were due to diet-induced changes present at the time of renal IRI (preconditioning) or differences in eating behavior after the injury leading to weight gain in the protected groups. To do this, we prevented access to food for one day following reperfusion in ad libitum fed animals as well as those fasted for two days prior to bilateral renal IRI. Although both groups continued to lose weight the first day after surgery (Fig 3A), animals fasted for two days prior to IRI had a significant survival advantage (Fig 3B) and significantly better renal function on the second day following the injury (Fig 3C). We cannot rule out the possibility that differential feeding after this initial 24 hour post-operative period, or other physiological post-operative differences between fasted and fed mice, contribute to the observed protection in the fasted group. However, our data are consistent with a substantial impact of diet prior to the onset of injury. We thus conclude that the impact of diet on the ability to withstand renal IRI is largely due to a preconditioning effect. The best known preconditioning technique is ischemic preconditioning, in which brief periods of ischemia are protective against longer, subsequent bouts of ischemia (Murray et al. 1986). Hereafter we refer to the effects observed here as dietary preconditioning.
Figure 3. Protection is a preconditioning effect.
A. Black bars indicate periods of free access to chow; grey bars indicate periods without access to chow. Body weights of the two groups at the time of surgery (day 0) are indicated. Error bars indicating SEM are contained within the symbols. B. Survival following renal IRI; fasted animals retained their survival advantage (p < 0.02) despite lack of refeeding for one day following renal IRI.
C. Kidney function as measured by serum urea levels before and after IRI. Asterisk indicates a significant difference between the fasted and ad libitum fed groups (p<0.05) on the second day following surgery.
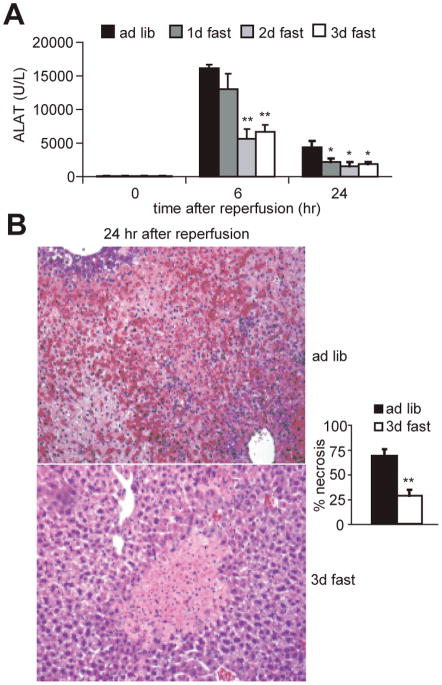
To find out if the protection afforded by brief fasting against IRI was specific to the kidney or more broadly applicable, we used a model of liver IRI. 70% hepatic ischemia was induced by clamping the portal vein, hepatic artery and bile duct to the left and median hepatic lobes for 75 minutes. Because no mortality is associated with this model, we monitored ischemic liver damage by measuring the release of the liver-specific enzyme ALAT from dead or damaged cells into the blood for up to 24 hours (Fig 4A). Six hours after reperfusion, serum ALAT levels were elevated above baseline in all groups. However, they were significantly lower in the 2 and 3 day fasted groups than in the ad libitum fed group. Twenty-four hours after reperfusion, serum ALAT levels were significantly lower in each of the fasted groups. Histological sections prepared 24 hours after reperfusion showed less hemorrhagic necrosis in the 3 day-fasted group than in the ad libitum fed group (Fig 4B). Thus as in the renal ischemia model, 1–3 days of water-only fasting significantly protected against ischemic damage to the liver.
Figure 4. Dietary preconditioning in the liver.
A. Reduced injury markers upon liver IRI in fasted mice. Mice (5–8 animals per group) were fasted for the indicated times prior to induction of 75 minutes of warm ischemia to the liver. Serum concentration of the liver-specific enzyme alanine aminotransferase (ALAT) indicative of liver damage was measured at the indicated times following reperfusion. Asterisks indicate the significance of the difference as compared to the ad libitum group at the same time point using a Mann-Whitney U-test (* p<0.05; ** p<0.01).
B. Representative hematoxylin/eosin-stained liver sections from mice 24 hours after reperfusion Note the large areas of hemorrhagic necrosis (in red) in the mouse fed ad libitum prior to IRI and its relative absence in the mouse fasted for 3 days prior. Magnification 100X. Right: Quantification of hemorrhagic necrosis. Liver necrosis was scored blindly on a scale from 0–4, with 4 representing 100% of the area covered by hemorrhagic necrosis. Asterisks indicate the significance of the difference as compared to the ad libitum group (** p<0.01).
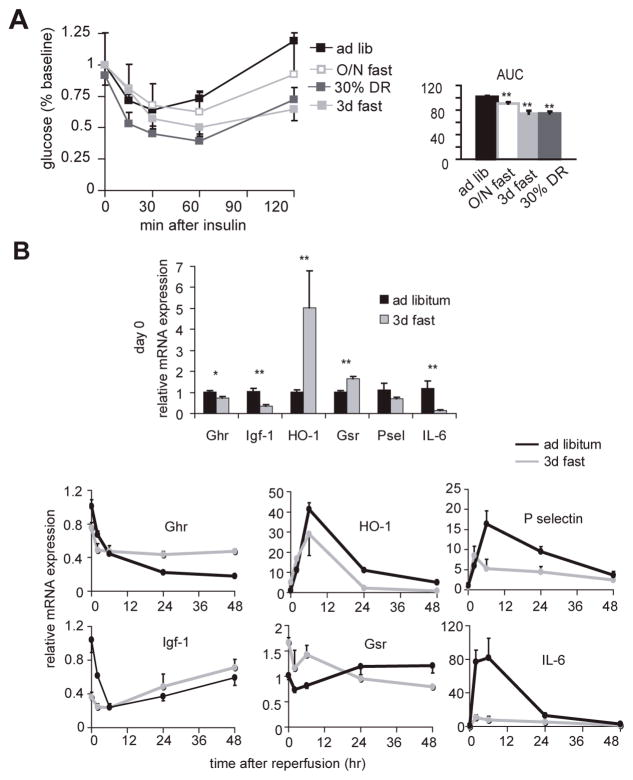
The mechanisms underlying the benefits of long-term DR, including resistance to IRI and extended longevity, remain unclear. Attenuation of oxidative stress, upregulation of stress proteins and reduced inflammation have emerged as potential mechanisms of resistance to heart and brain ischemia by long-term DR (Yu and Mattson 1999; Chandrasekar et al. 2001; Ahmet et al. 2005), while improved insulin sensitivity, reduced insulin/insulin-like growth factor (IGF)-1 signaling, upregulation of stress proteins, reduced mitochondrial free radical production and reduced inflammation are thought to contribute to extended longevity by long-term DR (Gredilla and Barja 2005; Sinclair 2005). We asked if dietary preconditioning by short-term DR (defined here as 4 weeks or less) and/or fasting could function via these candidate mechanisms. We first measured insulin sensitivity of animals fasted for 1 or 3 days or 30% restricted for 4 wks relative to ad libitum fed mice by injecting a bolus of insulin into the intraperitoneal cavity and measuring changes in blood glucose over time. The area under each curve is inversely proportional to that group’s ability to clear glucose in response to insulin challenge. Fasting and short-term DR resulted in improved insulin sensitivity relative to ad libitum fed mice, correlating with improved outcome following renal IR among our experimental groups (Fig 5A). Fasting improved insulin sensitivity in a dose-dependant manner from 1 to 3 days, with 3 days leading to an equivalent area under the curve as 4 weeks of DR.
Figure 5. Insulin and IGF-1 signaling in dietary preconditioning.
A. Improved insulin sensitivity in preconditioned mice. Whole blood glucose levels at the indicated timepoints following intraperitoneal injection of insulin into animals following the indicated preconditioning regimens. Right: area under the curves (AUC). Statistically significant differences relative to the ad libitum group are indicated by asterisks (** p<0.01; * p<0.05).
B. Differential expression of markers of antioxidant protection, inflammation and GH/IGF-1 axis. Changes in steady state mRNA levels of the indicated genes at baseline (bar graph, top) and over a two day time course following reperfusion (line graphs, bottom) as determined by quantitative real-time PCR. All data points are expressed relative to the ad libitum group at t=0 prior to renal IR. Each data point represents the mean expression value from 5 animals. HO-1, hemeoxygenase-1; Gsr, glutathione reductase; IL-6, interleukin-6; Ghr, growth hormone receptor; Igf-1, insulin-like growth factor-1. Top: asterisks indicate the significance of the difference as compared to the ad libitum group (* p<0.05; ** p<0.01).
We next analyzed components of the insulin/IGF-1 signaling pathway and the inflammatory response on the transcriptional level. At baseline, levels of kidney growth hormone receptor (Ghr) mRNA were significantly reduced in fasted relative to ad libitum fed animals (Fig 5B). Transcription of Igf-1 is dependent on binding of growth hormone to its cognate receptor, Ghr. Similar to Ghr, Igf-1 mRNA was significantly reduced in the fasted group (Fig 5B). Markers of antioxidant protection, including the inducible form of hemeoxygenase 1 (HO-1) and glutathione reductase (Gsr), were significantly elevated on the transcriptional level in fasted relative to ad libitum fed animals (Fig 5B). Following renal IRI, Ghr and Igf-1 mRNA levels fell below baseline in both groups. Interestingly, their decline was proportionately less in the fasted group than in the ad libitum group. Also, relative to baseline, the levels 48 hrs after reperfusion were higher in the fasted group than in the ad libitum group. HO-1 was strongly induced in both groups following ischemia, but to significantly lower levels and with a more rapid return to baseline in the fasted group. Gsr mRNA levels fell in the fasted group and increased in the fed group over the 48-hr time course following reperfusion. Markers of inflammation including the proinflammatory cytokine interleukin-6 and the neutrophil-recruiting endothelial adhesion molecule P-selectin were upregulated on the transcriptional level following IRI in both groups with similar kinetics, but to a significantly lower degree in the fasted group (Fig 5B).
We turned to a global approach to quantify the degree to which changes in gene expression due to fasting and short-term DR overlap with each other and with long-term DR. To this end, we compared kidney transcriptomes of animals preconditioned with 4 weeks 30% DR or 3 days fasting to a common group of ad libitum fed animals (n=3 animals per group) using Affymetrix arrays with 45,101 unique probe sets representing 36,431 target genes. 642 (fasted) and 161 (short-term DR) probe sets were significantly differentially regulated vs. the ad libitum control group using the criteria of fold change >1.5 with a p value of <0.001 (Fig 6A). 24 probe sets were common to both (Table 1), representing 14% and 4% of the total significant DR and fasted group probe sets, respectively. 79% of these changes occurred in the same direction, with a Pearson’s correlation of 0.529 and a Spearman’s rho of 0.464. Notable in this group of genes is a number involved in lipid metabolism (Lpl, Acsm3, Cyp51, Decr2, and Ces1), organelle/membrane trafficking (Ccdc91, Vsp8 and Bnip1) and protein turnover (Cndp2, Wdr40b, Rnf180 and Mmp13).
Figure 6. Global transcriptional changes upon short-term dietary restriction and fasting.
A. Venn diagram representing numbers and overlap of probesets significantly differentially regulated in kidney as a result of dietary preconditioning (4 wk 30% DR or 3 days fasting, FA) as compared to ad libitum fed controls. Significance cutoffs were set by fold change of greater than 1.5 and p value of <0.001.
B, C. All Gene Ontology - Biological Processes (B) and GENMAPP Pathway (C) gene sets over-represented within either the short-term DR or fasted vs. ad libitum data sets were aligned according to Z score. Red, green and black indicate upregulation, downregulation and or no change, respectively, of that biological process due to the given dietary treatment relative to ad libitum feeding. Gene sets are ordered by Z score of the 30% DR group. Note that all gene sets significantly over-represented in either treatment group (DR or FA) are included in the heat maps, and that those gene sets significantly over-represented in both treatment groups are in bold.
D. Similarity to long-term DR. Heat map of gene expression changes in a pre-defined set of 28 genes comprising a common transcriptional signature of DR across multiple mouse tissues (Swindell 2008). Long-term DR probesets are indicated either as up (red) or down (green) without regard to magnitude of expression; probesets corresponding to short-term DR and fasted groups are colored as in B. Significant genes from the short-term DR and fasted data sets as defined by fold change > 1.5 and p value <0.001 are in bold, with crosses and asterisks corresponding to short-term DR and fasting, respectively.
Table 1.
24 genes significantly differentially regulated in both short-term DR and fasted (FA) groups with fold changes >1.5 and p values <0.001. Expression level indicates the group geometric mean of the RMA normalized Affymetrix data, ordered by the ad libitum (AL) group.
| Gene | Symbol | Expression level
|
Fold change
|
Putative Function | |||
|---|---|---|---|---|---|---|---|
| AL | DR | FA | DR | FA | |||
| CNDP dipeptidase 2 (metallopeptidase M20 family) | Cndp2 | 14684 | 7766 | 3922 | −1.9 | −3.7 | proteolysis of nonspecific dipeptides in cytosol |
|
| |||||||
| lipoprotein lipase | Lpl | 10727 | 6045 | 5504 | −1.8 | −1.9 | triacylglycerol hydrolysis, lipoprotein uptake |
|
| |||||||
| acyl-CoA synthetase medium-chain family member 3 | Acsm3 | 7964 | 3417 | 3306 | −2.3 | −2.4 | activation of fatty acids for anabolism or catabolism |
|
| |||||||
| dopa decarboxylase | Ddc | 2558 | 1241 | 6356 | −2.1 | 2.5 | catecholamine biosynthesis |
|
| |||||||
| solute carrier family 22 member 7 | Slc22a7 | 2231 | 859 | 276 | −2.6 | −8.1 | bidirectional facilitative cGMP transporter |
|
| |||||||
| flavin containing monooxygenase 2 | Fmo2 | 2100 | 3792 | 3450 | 1.8 | 1.6 | oxidation of xenobiotics |
|
| |||||||
| cytochrome P450, family 51 | Cyp51 | 1731 | 1010 | 425 | −1.7 | −4.1 | key demethylase enzyme in all sterol biosynthesis |
|
| |||||||
| coenzyme Q10 homolog B (S. cerevisiae) | Coq 10b | 974 | 2069 | 453 | 2.1 | −2.2 | ubiqinone binding protein in yeast |
|
| |||||||
| solute carrier family 6, member 6 | Slc6a6 | 811 | 2177 | 2302 | 2.7 | 2.8 | taurine transporter |
|
| |||||||
| vacuolar protein sorting 8 homolog (S. cerevisiae) | Vps8 | 698 | 437 | 170 | −1.6 | −4.1 | endosomal/lysosomal biogenesis |
|
| |||||||
| tetraspanin 4 | Tspan4 | 643 | 1186 | 1119 | 1.8 | 1.7 | cell surface transmembrane integrin signaling |
|
| |||||||
| 2-4-dienoyl-Coenzyme A reductase 2, peroxisomal | Decr2 | 426 | 678 | 876 | 1.6 | 2.1 | peroxisomal oxidation of unsaturated fatty acids |
|
| |||||||
| RIKEN cDNA D630039A03 gene | D630039A03Rik | 323 | 156 | 618 | −2.1 | 1.9 | unknown |
|
| |||||||
| RIKEN cDNA 2310028N02 gene | 2310028N02Rik | 286 | 177 | 184 | −1.6 | −1.6 | transmembrane protein |
|
| |||||||
| carboxylesterase 1 | Ces 1 | 249 | 87 | 74 | −2.9 | −3.4 | detoxification of xenobiotics, cholesterol metabolism |
|
| |||||||
| coiled-coil domain containing 91 | Ccdc91 | 245 | 136 | 130 | −1.8 | −1.9 | sorting of acid hydrolase to endosomes |
|
| |||||||
| BCL2/adenovirus E1B interacting protein 1, NIP1 | Bnip 1 | 187 | 119 | 115 | −1.6 | −1.6 | anti-apoptotic factor/ER membrane fusion |
|
| |||||||
| mannan-binding lectin serine peptidase 1 | Masp 1 | 185 | 303 | 118 | 1.6 | −1.6 | complement activation and immune response |
|
| |||||||
| aryl hydrocarbon receptor nuclear translocator-like | Arntl (Bmal1) | 107 | 56 | 219 | −1.9 | 2.0 | transcription factor required for circadian rhythmicity |
|
| |||||||
| WD repeat domain 40B | Wdr40b | 80 | 45 | 48 | −1.8 | −1.7 | associated with DDB1/Cullin 4 ubiquitin ligase |
|
| |||||||
| matrix metallopeptidase 13 | Mmp 13 | 48 | 25 | 18 | −1.9 | −2.7 | breakdown of extracellular matrix |
|
| |||||||
| RIKEN cDNA 0610007P08 gene | 0610007P08Rik | 37 | 23 | 21 | −1.6 | −1.7 | possible DNA damage response |
|
| |||||||
| ring finger protein 180 | Rnf180 | 37 | 18 | 15 | −2.1 | −2.5 | membrane bound ubiquitin ligase |
|
| |||||||
| similar to MOSC domain-containing protein 1 | LOC100045982 | 30 | 69 | 642 | 2.3 | 21.1 | possible molybdenum cofactor biosythesis |
In light of this modest number of overlapping genes between dietary groups, we next asked which pathways were significantly affected by short-term DR and fasting, and how many were common to both. To this end, we looked for over-representation of pre-defined gene sets within each data set genes using GAzer (Kim et al. 2007) with a false discovery rate of q<0.05. Within the Gene Ontology (GO) category of Biological Processes (GO-BP), 43 and 27 genes sets were significantly over-represented in the DR and fasted groups, respectively. 13 of these were common to both treatments, with Z scores indicating the same directionality in all 13 gene sets. In GENMAPP Pathway, 19 and 9 gene sets were significantly over-represented in the DR and fasted groups, respectively, with 4 of the 6 in common in the same direction. Heat maps of these enriched gene sets in both treatment groups ordered by Z score of the DR group are shown in Fig 6B,C. Similar overlaps were seen within other sets of pre-defined gene sets, including GO Molecular Functions (GO-MF; 40 DR and 25 fasted significant gene sets, 12 common to both, all in the same direction) GO Cellular Component (GO-CC; 18 DR and 7 fasted significant gene sets, 3 common to both, all in the same direction) and Interpro (41 DR and 49 fasted significant gene sets, 19 in common, all in the same direction). Thus, despite only modest overlap on the level of individual genes, 53 out of the 161 and 117 significant gene sets in the short-term DR and fasted groups, respectively, were common to both treatments, with 51 of these in the same direction.
Amongst these pathways were many expected for nutrient/energy deprivation by either fasting or DR, including downregulation of fatty acid, cholesterol and steroid biosynthesis. Pathways previously reported to be downregulated upon dietary restriction, such as DNA repair (Stuart et al. 2004), were also downregulated (although not significantly) in the fasted group. In addition, shared pathways consistent with increased resistance to IRI were significantly over-represented, including increased GO-BP negative regulation of apoptosis (upregulated anti-apoptotic genes included angiopoietin-like 4 in both groups and Bcl2-like 1 in the fasted group), increased GO-MF glutathione transferase activity (increased Gstm1, Gstm3 in DR; increased Mgst-1, Gsta1, Gsta2, Gsta3, Gsto1, Gstt2, Gstm1 in FA) and decreased GO-BP complement activation. Of the 19 common significant pathways represented in Fig 6B, differences between dietary treatments were observed in only 2, Statin and Electron Transport Chain (ETC). In the ETC Pathway, for example, a number of genes encoding subunits of cytochrome c oxidase, NADH dehydrogenase and ATP synthase were significantly upregulated in DR and downregulated in FA, accounting for the difference in directionality. Although ROS production and/or detoxification is thought to be a key player in the anti-aging effects of DR, the role of mitochondrial respiration itself remains controversial (Kaeberlein et al. 2005). Further experiments will be required to test which if any of these pathways are involved in the beneficial effects of dietary preconditioning against IRI.
Finally, we asked if the effects of short-term DR and fasting on gene expression in the kidney were similar to what has been previously reported for long-term DR in other mouse tissues. For data regarding long-term DR, we turned to a meta-analysis covering ten mouse tissues subject to varying lengths (2d-2yr, average 6 mo) and severity (10–75%, average 36%) of DR (Swindell 2008). 28 genes that change significantly upon DR in at least 5 tissues were identified in this meta-analysis. Within this group of 28 genes/48 probe sets), 2 genes/2 probe sets (PER2, TSPAN4) from our DR data set and 8 genes/9 probe sets (CD74, COL3A1, TSPAN 4, FKBP5, RBM3, PECI, SERPINH1 and CDKN1A) from our fasted data set were significantly differentially regulated in the kidney (fold change >1.5, p<0.001). This degree of enrichment makes it amongst the most significant of any pre-defined gene set tested (p= 0.013 and p =2.353e-8 for DR and fasted, respectively, by Fisher’s exact test). A heat map representing expression levels of probe sets corresponding to the 28 DR-associated genes is presented in Fig 6D.
Discussion
Timing of onset of benefits of DR
Voluntary adherence to a restricted diet is difficult for most people. Potential benefits of DR requiring long-term application, such as extended longevity, are thus considered moot in a clinical setting. However, extended longevity is only one of the potential benefits of DR (albeit a widely popularized one). DR also increases acute stress resistance in most organisms tested, including mammals. For example, DR lasting between 3 months and 1 year mitigates injury in rodent models of cardiac and cerebral ischemia (Yu and Mattson 1999; Chandrasekar et al. 2001; Ahmet et al. 2005). What is currently not known is the length of restriction required for the onset of such benefits. Here we show that as little as 2–4 weeks of 30% reduced daily feeding in mice led to significant protection against renal IRI. Interestingly, there was no increase in protection between 2 and 4 weeks of DR, suggesting that the maximal protection afforded by these short-term treatments was already reached by 2 weeks of DR. Whether or not longer periods of restriction will further increase protection remains to be experimentally determined.
In rodents, longevity extension by DR is roughly proportional to the amount of energy restriction (0–65%) up to the point of starvation (Weindruch et al. 1986; Merry 2002). Beyond this point, restriction can lead to irreversible consequences and is eventually fatal. However, 100% restriction (water-only fasting) for shorter periods of time is possible without irreversible side-effects. We found that 3 days of water-only fasting led to protection against renal ischemic injury similar in magnitude to 2–4 weeks of 30% DR. Significant benefits were observed with respect to both survival and kidney function; however, the kinetics of onset and loss differed between these endpoints. Significant survival benefits occurred after a single overnight fast, while protection against renal dysfunction increased each fasting day for up to three days. Similarly, functional protection afforded by a 3 day fast was rapidly lost within hours of refeeding despite lingering survival benefits for up to several days. The reason for these apparent discrepancies in timing of onset and loss of benefits is currently not known. One possibility is that death and kidney dysfunction, although both initiated by renal IRI, have partially different causes. For example, mortality may be caused by reperfusion injury to distant organs such as the heart, lungs and brain (Kelly 2003) in addition to renal failure. Fasting may more rapidly affect the body’s ability to withstand remote ischemic injury than acute renal ischemic injury. Another, non-mutually exclusive possibility is that different mechanisms of protection exist and that they are gained and lost at different rates. For example, transcriptional upregulation of stress resistance genes including HO-1 and Gsr may require several days of reduced insulin/IGF-1 signaling, but be rapidly lost upon the return of such signaling. Future experiments will be required to determine molecular mechanisms underlying individual benefits and their timing of onset and loss as a function of different dietary regimens.
This is the first demonstration of the rapid onset of resistance to renal ischemic damage by short-term DR and fasting. However, it is consistent with a larger body of work demonstrating rapid onset of benefits of energy/nutrient deprivation, including both fasting and DR, in different organisms using different endpoints. For example, in fruit flies maximal effects on daily mortality rate are achieved within one to three days of DR (Mair et al. 2003). Resistance to starvation stress is also increased by four days of DR and scales with the percent restriction (Chippindale et al. 1993). In mice, 40% DR for 12–15 days is effective at preventing growth of tumor xenografts (Kalaany and Sabatini 2009). DR initiated late in life rapidly shifts liver gene expression towards a long-term DR profile, beginning in a little as 2 weeks, and impacts longevity within two months (Dhahbi et al. 2004). Two weeks of DR also leads to feminization of liver gene expression in males (Estep et al. 2009), an intriguing finding in light of the dramatically increased resistance of females to renal IRI relative to males in both humans and mice (Kher et al. 2005). Shorter periods of more severe restriction have also proven efficacious in protecting against acute stress. For example, fasting for 3–4 days improves organ and animal survival in rodent models of orthotopic liver transplantation (Sumimoto et al. 1993) and heterotopic heart transplantation (Nishihara et al. 1997). Finally, two days of water-only fasting protects mice against the toxic effects of chemotherapeutic agents (Raffaghello et al. 2008). Although most of these studies were not designed to interrogate the time on a restricted diet required for the onset of maximal benefit, taken together with our own studies they suggest that benefits of nutrient/energy restriction measured by a variety of outcomes, including acute stress resistance, do not require long-term application.
Potential overlap between mechanisms of protection by fasting, short-term and long-term DR
One of the consequences of nutrient/energy deprivation is reduced insulin/IGF-1 signaling. Reduced signaling through these pathways increases stress resistance and extends longevity in part through activation of transcription factors leading to increased expression of stress resistance genes (Tatar et al. 2003). In rodents, improved insulin sensitivity is indicative of reduced baseline insulin signaling and is observed in both genetic and dietary models of extended longevity and increased stress resistance. Relative to ad libitum fed mice, we observed improved insulin sensitivity upon both fasting and short-term DR. We also observed reduced levels of growth hormone receptor and IGF-1 mRNAs in the kidney itself, as well as upregulation of genes involved in antioxidant defense. These data are consistent with a model of increased stress resistance by both fasting and short-term DR involving reduced insulin/IGF signaling and concomitant upregulation of cellular stress resistance mechanisms. As a consequence, oxidative injury, cell death and inflammation are all reduced subsequent to ischemic injury.
In a variety of experimental models, reduced signaling through insulin/insulin-like peptides results in extended longevity and increased stress resistance (Brown-Borg 2006). This may seem at odds with the well-documented pro-growth and pro-survival effects these factors have in other settings, for example in response to ischemic insult to the brain, heart or kidney (Miller et al. 1992; Guan et al. 2003; Takeda et al. 2006; Suleiman et al. 2007). Here we observed improved insulin sensitivity and reduced Ghr and Igf-1 mRNA in fasted mice consistent with reduced signaling through these pathways at baseline prior to IRI. Interestingly, we also observed a relative increase in mRNA levels of Ghr and Igf-1 after injury in the fasted group. Although further experiments are required to determine actual levels of GH/IGF-1 signaling, the present data are consistent with increased signaling through these pathways after ischemic insult and better survival. Based on these data, we hypothesize that the benefits of reduced and increased insulin/IGF signaling can be separated by time relative to an acute injury, and are thus not mutually exclusive. According to this hypothesis, reduced IGF-1 signaling as a result of DR or fasting prior to ischemic insult may have two beneficial effects: upregulation of stress resistance resulting in less initial damage, and improved IGF-1 sensitivity resulting in increased survival signaling after injury.
In lower organisms, the genetic requirements for lifespan extension and stress resistance are more extensively characterized than in mammals, and appear to vary depending on the type of DR method employed. In worms, for example, the source of bacteria (liquid culture vs. solid agar plates) and the timing of onset of DR can both affect the genetic requirements for longevity extension (Greer et al. 2007). Here we used two distinct dietary interventions, fasting and short-term DR, to modulate resistance to ischemic injury and found similar beneficial effects with both. On the level of global changes in the kidney transcriptome, 31% of the significant short-term DR and 49% of the significant fasting annotated gene sets identified in GO-BP, GO-MF, GO-CC, GENMAPP Pathway and Interpro were common to both treatment groups. Of the 53 significant gene sets in common, 51 were changed in the same direction. Furthermore, gene expression markers identified previously in a meta-analysis of mostly long-term DR studies of multiple mouse organs (Swindell 2008) were significantly enriched in our kidney data set from both short-term DR and fasted animals. Taken together, these data are consistent with an overlapping transcriptional response to fasting, short-term DR and long-term DR in the kidney. However, they are also consistent with different genetic requirements for different DR regimens as in lower organisms. Future experiments will be required to determine which of these changes, if any, underlie the benefits of dietary restriction on acute stress resistance.
Finally, it will be of great future interest to elucidate the relationship between acute stress resistance and other benefits of DR such as extended longevity. Without any detailed understanding of the mechanism (or mechanisms) underlying either of these benefits, the relationship between them remains only correlative in nature. It is easy to envisage how improved stress resistance could extend longevity, for example by increasing the likelihood of surviving a heart attack or stroke on any given day. What remains unclear is if, or how, this relates to the reduction in the rate of aging achieved by DR.
Fasting in DR and acute stress resistance
The role of periods of fasting in the beneficial action of DR remains unclear (Mattson 2005). DR regimens in rodents involve extended periods of fasting between meals, as animals are hungry and consume their food allotment quickly when fed. The length of these periods is not widely reported and likely depends both on the percent restriction as well as the frequency of meals (typically once daily to thrice weekly). Intermittent fasting regimens with ad libitum feeding between periods of fasting (for example, every other day fasting or 4 days fasting every two weeks) have beneficial effects in rodents similar to DR, including extended lifespan (Goodrick et al. 1982; Sogawa and Kubo 2000) and increased stress resistance (Anson et al. 2003). In some strains, intermittent fasting is associated with reduced total food intake and reduced body weight typical of DR (Varady and Hellerstein 2007), while in others, it produces tangible health benefits in the absence of weight loss (Sogawa and Kubo 2000; Anson et al. 2003). This suggests that periods of fasting rather than reduced overall calorie intake per se may underlie some of the benefits of DR. On the other hand, altering the temporal pattern of feeding of a restricted diet from once to twice daily, while affecting circadian rhythmicity, has no significant impact on lifespan (Masoro et al. 1995). This suggests that overall nutrient/energy restriction is more important than timing of food intake at least for longevity extension by DR. Nevertheless, these experiments did not rule out the potential role of fasting between meals, as both groups still underwent extended periods of fasting relative to the ad libitum group (Masoro 2004). Experimental separation of reduced nutrient/energy intake from periods of fasting in experimental rodents remains challenging, and further experiments will be required to resolve the contribution of periods of fasting, if any, to the benefits of DR. Our data are consistent with a role of fasting in at least one of these benefits, acute stress resistance, during the initiation phase of DR.
The benefits of fasting have been known at least since the time of Hippocrates, but the practice is largely absent from Western medicine today. Instead, it is often associated with malnutrition, a risk factor for postoperative survival (Studley 1936), wound healing, infection and multiple organ failure (Chung 2002). One of the few modern clinical applications of fasting is to reduce the risk of pulmonary aspiration of regurgitated stomach contents prior to operations involving anesthesia. The origins in the 1960s of the standard “nil by mouth after midnight” preoperative fast are somewhat obscure (Maltby 2006), and today even this relatively short fast is perceived as overcautious and possibly detrimental to patient subjective well-being and postoperative recovery (Diks et al. 2005). Current more liberal guidelines allow consumption of solids up to six hours prior to surgery and recommend consumption of liquids, including carbohydrate-rich beverages, up to two hours prior to surgery (Soreide et al. 2005). Our data suggest that slightly longer periods of fasting, or short periods of DR, prior to surgery may be beneficial for an entirely different purpose – protection against certain types of acute organ stress. Fasting may thus represent a non-invasive, cost-free method of protecting against multiple types of acute stress, including surgical ischemia reperfusion injury unavoidably encountered in elective surgeries including living-donor organ transplantation, cardiac surgery, vascular surgery and liver resection. It remains to be seen if the benefits of fasting and short-term DR and their rapid onset observed in rodents will translate to humans. However, the conservation of rapid onset of DR benefits between flies (Mair et al. 2003) and rodents, combined with the efficacy of DR in improving markers of healthspan in both non-human primates (Colman et al. 2008; Colman et al. 2009) and humans (Fontana and Klein 2007) suggests that it might.
Experimental procedures
Mice
Male C57BL/6J mice in the age/weight range of 22–25 grams/10–14 weeks were purchased from Charles River Laboratories, Maastricht, the Netherlands. Animals were kept under standard laboratory conditions (temperature 20–24°C, relative humidity 50–60%, 12 h light/12 h dark) with 3–4 animals per cage and allowed free access to water and food (Hope Farms, Woerden, The Netherlands) except where noted. All experiments were performed with the approval of the appropriate local ethical board.
Dietary regimens
The amount of food eaten ad libitum was approximately 3.5 gram/day as determined by weighing the remaining food on a daily basis for one week. DR was applied for 2–4 weeks by feeding mice 70% of this amount on a daily basis. Fasting was applied by transferring mice to a fresh cage without food for 1–3 days. Despite significant weight loss as a result of short-term DR and fasting, no morbidity or mortality was observed as a function of the diets alone.
Renal ischemia model
Mice were anaesthetized by isoflurane inhalation (5% isoflurane initially and then 2–2.5% with oxygen for maintenance). Body temperature was maintained by placing the animals on heating pads until recovery from anesthesia. Following a midline abdominal incision, the left renal pedicle was localized and the renal artery and vein were dissected. An atraumatic microvascular clamp was used to occlude the left kidney for 37 minutes. For bilateral occlusion, the procedure was repeated immediately on the right kidney. After inspection for signs of ischemia (purple color), the wound was covered with phosphate-buffered saline (PBS)-soaked cotton and the animal placed under an aluminum foil blanket to maintain body temperature. After release of the clamp, restoration of blood-flow was inspected by return of the kidney to normal color. In the case of unilateral occlusion, a contralateral nephrectomy was performed immediately following clamp release. The abdominal wound was closed in two layers using 5/0 sutures. Animals were given 0.5 mL PBS subcutaneously for maintenance of fluid balance and kept warm under a heat lamp. All animals were observed to have regained consciousness before moving their cages from the operating room to the stable.
Kidney function analysis
Unlabeled DMSA kits were purchased from GE Healthcare (Roosendaal, the Netherlands) and radiolabeled with 99mTc according to manufacturer’s instructions. Mice were injected in a lateral tail vein with 30 MBq 99mTc-DMSA. Four hours post injection the mice were sacrificed, the kidney was removed, and the absorbed radioactivity was measured in a gamma counter (Perkin Elmer, Groningen, the Netherlands) and expressed as percentage of injected dose per kidney (%ID/kidney).
Liver ischemia model
Liver IRI was performed by visualizing the liver hilus and clamping the portal vein, hepatic artery and bile duct to the left and median hepatic lobes with an atraumatic clamp for 75 minutes. In this model, 70% of the liver tissue becomes ischemic, and blood outflow from the small intestine is preserved through the right anterior and caudate liver lobes. Mortality associated with this amount of ischemic damage to the liver was not observed. Liver samples were fixed in formalin for 24 hr prior to embedding in paraffin. 4 μm sections were cut and stained with hematoxylin and eosin. Liver necrosis was scored blindly on a scale from 0–4, with 4 representing 100% of the area covered by hemorrhagic necrosis.
Serum measurements
Blood samples were collected by retro-orbital puncture. Serum urea and creatinine levels were measured using QuantiChrom assay kits based on the improved Jung and Jaffe methods, respectively (DIUR-500 and DICT-500, Gentaur, Brussels, Belgium). Serum ALAT levels were determined using an ELAN analyzer (Eppendorf Merck, Hamburg, Germany) with Ecoline S+ reagents (Diagnostic Systems GmbH, Holzheim, Germany) according to manufacturer’s instructions. Serum LDH levels were determined using Ecoline S+ reagents according to manufacturer’s instructions in a 96-well format on a Varioskan microplate reader (Thermo Scientific).
Histology
Organs were harvested, fixed for 24 hr in formalin and embedded in paraffin. 3 μm sections were stained with hematoxylin and eosin. Tubular injury was assessed in a blind fashion on a five point scale as described previously (Leemans et al. 2005).
Insulin sensitivity tests
Following baseline blood glucose determination from tail blood of conscious, restrained mice, animals were injected with a bolus of insulin (Novorapid; 0.75 U/kg body weight) into the intraperitoneal cavity. Blood glucose determinations were performed at the indicated times after injection using a HemoCue glucose 201 RT blood glucose analyzer (HemoCue, Ängelholm, Sweden) according to the manufacturer’s instructions.
Quantitative real time PCR
Total RNA was extracted from frozen kidney tissue using Ambion mirVana miRNA Isolation Kit and oligodT or hexamer-primed cDNA synthesized using SuperScript II (Invitrogen) according to the manufacturer’s instructions. Quantitative real-time PCR was performed using a MyIQ (BioRad) with SYBR Green incorporation. Relative expression was calculated using the equation 1.8−(ΔCt sample − ΔCt control) (Pfaffl 2001). Each sample was tested in duplo at least two times.
Microarrays
Purified total RNA extracted from frozen kidneys (three per group) was used as template to generate biotin labeled cRNA and hybridized to 430-2.0 GeneChips according to the manufacture’s protocol (Affymetrix). Raw data was RMA normalized (Irizarry et al. 2003) and assessed for differential gene expression using limma (Smyth 2004). Annotated pathway over-representation was performed using GAzer (Kim et al. 2007). Fisher’s Exact test was performed using the web application available at http://www.langsrud.com/fisher.htm. Spearman’s rho and Pearson’s correlation coefficients were calculated using SPPS11. Affymetrix 430-2.0 probe sets representative of the 28 common dietary restricted genes (Swindell 2008) were obtained from Ensembl (http://www.ensembl.org/), based on the NCBI m37 mouse assembly; expect for MGI:1924575 which was obtained from the Mouse Genome Informatics data base (http://www.informatics.jax.org/) given the absence of annotations in Ensembl for this transcript at the time of submission.
Statistics
Data are expressed as the mean ± SEM. Statistical analyses of data on urea, creatinine, histomorphology, immunohistochemistry and immunoblot was preformed using a Student’s T test unless otherwise indicated. Survival was analyzed by Kaplan-Meier (SPSSv11). Area under the curve was calculated using GraphPad Prism 4.0, and the significance calculated using a Student’s T test.
Acknowledgments
Thanks to Joris Pothof, Karl Brand, Bas Zwaan, Rudi Westendorp, Henk Roest and Steven Russell for discussions and critical reading of the manuscript, and to Paula van Heijningen for technical assistance. This work was supported by grants from the Netherlands Organization for Scientific Research (Healthy Ageing stimulation grant 05040202 from the Netherlands Genomics Initiative), the Netherlands Organization for Health Research (Research Institute of Diseases in the Elderly 60-60400-98-004), the United States National Institutes of Health (1PO1 AG17242-02), the European Commission RISC-RAD contract number FI6R-CT-2003-508842, the Association of International Cancer Research (05-0280) and the Ellison Medical Foundation (5379825-01). M.V. was supported by a grant from the Dutch Kidney Foundation (C07-2206).
References
- Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112:3115–21. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–20. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop NA, Guarente L. Genetic links between diet and lifespan: shared mechanisms from yeast to humans. Nat Rev Genet. 2007;8:835–44. doi: 10.1038/nrg2188. [DOI] [PubMed] [Google Scholar]
- Brown-Borg HM. Longevity in mice: is stress resistance a common factor? AGE. 2006;28:145–162. doi: 10.1007/s11357-006-9003-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandrasekar B, Nelson JF, Colston JT, Freeman GL. Calorie restriction attenuates inflammatory responses to myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;280:H2094–102. doi: 10.1152/ajpheart.2001.280.5.H2094. [DOI] [PubMed] [Google Scholar]
- Chippindale AK, Leroi AM, Kim SB, Rose MR. Phenotypic plasticity and selection in Drosophila life-history evolution. I. Nutrition and the cost of reproduction. Journal of Evolutionary Biology. 1993;6:171–193. [Google Scholar]
- Chung A. Perioperative nutrition support. Nutrition. 2002;18:207–8. doi: 10.1016/s0899-9007(01)00760-2. [DOI] [PubMed] [Google Scholar]
- Colman RJ, Anderson RM, Johnson SC, Kastman EK, Kosmatka KJ, Beasley TM, Allison DB, Cruzen C, Simmons HA, Kemnitz JW, Weindruch R. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325:201–4. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colman RJ, Beasley TM, Allison DB, Weindruch R. Attenuation of sarcopenia by dietary restriction in rhesus monkeys. J Gerontol A Biol Sci Med Sci. 2008;63:556–9. doi: 10.1093/gerona/63.6.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comfort A. Effect of delayed and resumed growth on the longevity of a fish (Lebistes reticulatus Peters) in captivity. Gerontologia (Basel) 1963;8:150–155. doi: 10.1159/000211216. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Kim HJ, Mote PL, Beaver RJ, Spindler SR. Temporal linkage between the phenotypic and genomic responses to caloric restriction. Proc Natl Acad Sci U S A. 2004;101:5524–9. doi: 10.1073/pnas.0305300101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diks J, van Hoorn DE, Nijveldt RJ, Boelens PG, Hofman Z, Bouritius H, van Norren K, van Leeuwen PA. Preoperative fasting: an outdated concept? JPEN J Parenter Enteral Nutr. 2005;29:298–304. doi: 10.1177/0148607105029004298. [DOI] [PubMed] [Google Scholar]
- Estep PW, 3rd, Warner JB, Bulyk ML. Short-term calorie restriction in male mice feminizes gene expression and alters key regulators of conserved aging regulatory pathways. PLoS One. 2009;4:e5242. doi: 10.1371/journal.pone.0005242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S. Aging, adiposity, and calorie restriction. Jama. 2007;297:986–94. doi: 10.1001/jama.297.9.986. [DOI] [PubMed] [Google Scholar]
- Friedewald JJ, Rabb H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004;66:486–91. doi: 10.1111/j.1523-1755.2004.761_3.x. [DOI] [PubMed] [Google Scholar]
- Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth and life span in rats. Gerontology. 1982;28:233–41. doi: 10.1159/000212538. [DOI] [PubMed] [Google Scholar]
- Gredilla R, Barja G. Minireview: the role of oxidative stress in relation to caloric restriction and longevity. Endocrinology. 2005;146:3713–7. doi: 10.1210/en.2005-0378. [DOI] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17:1646–56. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J, Bennet L, Gluckman PD, Gunn AJ. Insulin-like growth factor-1 and post-ischemic brain injury. Prog Neurobiol. 2003;70:443–62. doi: 10.1016/j.pneurobio.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. Jama. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JC, Jaruga E, Repnevskaya MV, Jazwinski SM. An intervention resembling caloric restriction prolongs life span and retards aging in yeast. Faseb J. 2000;14:2135–7. doi: 10.1096/fj.00-0242fje. [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, Hu D, Kerr EO, Tsuchiya M, Westman EA, Dang N, Fields S, Kennedy BK. Increased life span due to calorie restriction in respiratory-deficient yeast. PLoS Genet. 2005;1:e69. doi: 10.1371/journal.pgen.0010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaany NY, Sabatini DM. Tumours with PI3K activation are resistant to dietary restriction. Nature. 2009;458:725–31. doi: 10.1038/nature07782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly KJ. Distant effects of experimental renal ischemia/reperfusion injury. J Am Soc Nephrol. 2003;14:1549–58. doi: 10.1097/01.asn.0000064946.94590.46. [DOI] [PubMed] [Google Scholar]
- Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR. Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res. 2005;67:594–603. doi: 10.1016/j.cardiores.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Kim SB, Yang S, Kim SK, Kim SC, Woo HG, Volsky DJ, Kim SY, Chu IS. GAzer: gene set analyzer. Bioinformatics. 2007;23:1697–9. doi: 10.1093/bioinformatics/btm144. [DOI] [PubMed] [Google Scholar]
- Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–29. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Leemans JC, Stokman G, Claessen N, Rouschop KM, Teske GJ, Kirschning CJ, Akira S, van der Poll T, Weening JJ, Florquin S. Renal-associated TLR2 mediates ischemia/reperfusion injury in the kidney. J Clin Invest. 2005;115:2894–903. doi: 10.1172/JCI22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 2000;289:2126–8. doi: 10.1126/science.289.5487.2126. [DOI] [PubMed] [Google Scholar]
- Mair W, Goymer P, Pletcher SD, Partridge L. Demography of dietary restriction and death in Drosophila. Science. 2003;301:1731–3. doi: 10.1126/science.1086016. [DOI] [PubMed] [Google Scholar]
- Maltby JR. Fasting from midnight--the history behind the dogma. Best Pract Res Clin Anaesthesiol. 2006;20:363–78. doi: 10.1016/j.bpa.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Subfield history: caloric restriction, slowing aging, and extending life. Sci Aging Knowledge Environ. 2003;2003:RE2. doi: 10.1126/sageke.2003.8.re2. [DOI] [PubMed] [Google Scholar]
- Masoro EJ. Caloric intake versus temporal pattern of food intake. Aging Clin Exp Res. 2004;16:423–4. doi: 10.1007/BF03327395. [DOI] [PubMed] [Google Scholar]
- Masoro EJ, Shimokawa I, Higami Y, McMahan CA, Yu BP. Temporal pattern of food intake not a factor in the retardation of aging processes by dietary restriction. J Gerontol A Biol Sci Med Sci. 1995;50A:B48–53. doi: 10.1093/gerona/50a.1.b48. [DOI] [PubMed] [Google Scholar]
- Mattson MP. The need for controlled studies of the effects of meal frequency on health. Lancet. 2005;365:1978–80. doi: 10.1016/S0140-6736(05)66667-6. [DOI] [PubMed] [Google Scholar]
- McCay CM, Crowel MF, Maynard LA. The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr. 1935;10:63–79. [PubMed] [Google Scholar]
- Merry BJ. Molecular mechanisms linking calorie restriction and longevity. Int J Biochem Cell Biol. 2002;34:1340–54. doi: 10.1016/s1357-2725(02)00038-9. [DOI] [PubMed] [Google Scholar]
- Miller SB, Martin DR, Kissane J, Hammerman MR. Insulin-like growth factor I accelerates recovery from ischemic acute tubular necrosis in the rat. Proc Natl Acad Sci U S A. 1992;89:11876–80. doi: 10.1073/pnas.89.24.11876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Nishihara V, Sumimoto R, Fukuda Y, Southard JH, Asahara T, Dohi K. Inhibition of warm ischemic injury to rat liver, pancreas, and heart grafts by controlling the nutritional status of both donor and recipient. Surg Today. 1997;27:645–50. doi: 10.1007/BF02388222. [DOI] [PubMed] [Google Scholar]
- Partridge L, Green A, Fowler K. Effects of egg-production and of exposure to males on female survival in Drosophila melanogaster. Journal of Insect Physiology. 1987;33:745–749. [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105:8215–20. doi: 10.1073/pnas.0708100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson A, Liu F, Adamo ML, Van Remmen H, Nelson JF. The role of insulin and insulin-like growth factor-I in mammalian ageing. Best Pract Res Clin Endocrinol Metab. 2004;18:393–406. doi: 10.1016/j.beem.2004.02.002. [DOI] [PubMed] [Google Scholar]
- Sinclair DA. Toward a unified theory of caloric restriction and longevity regulation. Mech Ageing Dev. 2005;126:987–1002. doi: 10.1016/j.mad.2005.03.019. [DOI] [PubMed] [Google Scholar]
- Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- Sogawa H, Kubo C. Influence of short-term repeated fasting on the longevity of female (NZB x NZW)F1 mice. Mech Ageing Dev. 2000;115:61–71. doi: 10.1016/s0047-6374(00)00109-3. [DOI] [PubMed] [Google Scholar]
- Soreide E, Eriksson LI, Hirlekar G, Eriksson H, Henneberg SW, Sandin R, Raeder J. Pre-operative fasting guidelines: an update. Acta Anaesthesiol Scand. 2005;49:1041–7. doi: 10.1111/j.1399-6576.2005.00781.x. [DOI] [PubMed] [Google Scholar]
- Stuart JA, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. Mitochondrial and nuclear DNA base excision repair are affected differently by caloric restriction. Faseb J. 2004;18:595–7. doi: 10.1096/fj.03-0890fje. [DOI] [PubMed] [Google Scholar]
- Studley HO. Percentage of weight loss: a basic indicator of surgical risk in patients with chronic peptic ulcer. JAMA. 1936;106:458–60. [PubMed] [Google Scholar]
- Suleiman MS, Singh RJ, Stewart CE. Apoptosis and the cardiac action of insulin-like growth factor I. Pharmacol Ther. 2007;114:278–94. doi: 10.1016/j.pharmthera.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Sumimoto R, Southard JH, Belzer FO. Livers from fasted rats acquire resistance to warm and cold ischemia injury. Transplantation. 1993;55:728–32. doi: 10.1097/00007890-199304000-00008. [DOI] [PubMed] [Google Scholar]
- Sun D, Muthukumar AR, Lawrence RA, Fernandes G. Effects of calorie restriction on polymicrobial peritonitis induced by cecum ligation and puncture in young C57BL/6 mice. Clin Diagn Lab Immunol. 2001;8:1003–11. doi: 10.1128/CDLI.8.5.1003-1011.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindell WR. Comparative analysis of microarray data identifies common responses to caloric restriction among mouse tissues. Mech Ageing Dev. 2008;129:138–53. doi: 10.1016/j.mad.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda R, Nishimatsu H, Suzuki E, Satonaka H, Nagata D, Oba S, Sata M, Takahashi M, Yamamoto Y, Terauchi Y, Kadowaki T, Kangawa K, Kitamura T, Nagai R, Hirata Y. Ghrelin improves renal function in mice with ischemic acute renal failure. J Am Soc Nephrol. 2006;17:113–21. doi: 10.1681/ASN.2004080626. [DOI] [PubMed] [Google Scholar]
- Tatar M, Bartke A, Antebi A. The endocrine regulation of aging by insulin-like signals. Science. 2003;299:1346–51. doi: 10.1126/science.1081447. [DOI] [PubMed] [Google Scholar]
- Varady KA, Hellerstein MK. Alternate-day fasting and chronic disease prevention: a review of human and animal trials. Am J Clin Nutr. 2007;86:7–13. doi: 10.1093/ajcn/86.1.7. [DOI] [PubMed] [Google Scholar]
- Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of ageing in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. Journal of Nutrition. 1986;116:641–654. doi: 10.1093/jn/116.4.641. [DOI] [PubMed] [Google Scholar]
- Weiss EP, Racette SB, Villareal DT, Fontana L, Steger-May K, Schechtman KB, Klein S, Holloszy JO. Improvements in glucose tolerance and insulin action induced by increasing energy expenditure or decreasing energy intake: a randomized controlled trial. Am J Clin Nutr. 2006;84:1033–42. doi: 10.1093/ajcn/84.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–9. [PubMed] [Google Scholar]