Fig. 1.
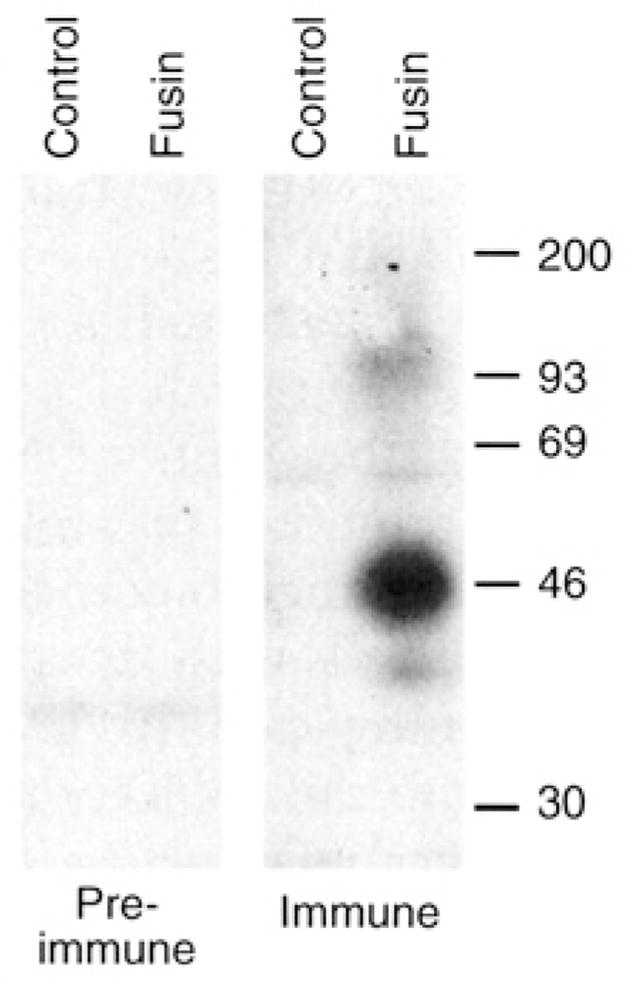
Protein immunoblot analysis of fusin produced by a recombinant vaccinia virus. BS-C-1 cells were infected with vCBYFI -fusin or with control virus WR (multiplicity of infection = 10). After overnight incubation at 37°C, the cells were washed twice with phosphate-buffered saline (PBS), pelleted, and lysed in buffer containing 1 % (v/v) Nonidet P-40,150 mM NaCl, and 10 mM tris (pH 7.4) plus protease inhibitors. The lysates were incubated 30 min at 4°C, then clarified by centrifugation at 10,000g for 5 min at 4°C. For protein immunoblot analysis, 10-μl aliquots of each lysate (representing ~5 × 104 cells) were mixed with 30 μl of 1 × reducing sample buffer supplemented with 8 M urea; the samples were incubated at 37°C overnight, then at 100°C for 3 min and subjected to SDS-polyacrylamide gel electrophoresis on 10% gels. Proteins were electrophoretically transferred to a nitrocellulose membrane, blocked in a solution containing 1% (w/v) bovine serum albumin and 0.15% (v/v) Tween-20, and incubated overnight with immune or pre-immune sera (1:200 dilution in blocking buffer). Bound antibodies were detected with [125I]Protein A (Amersham) and autoradiography. Size markers are shown on the right in kilodaltons.
