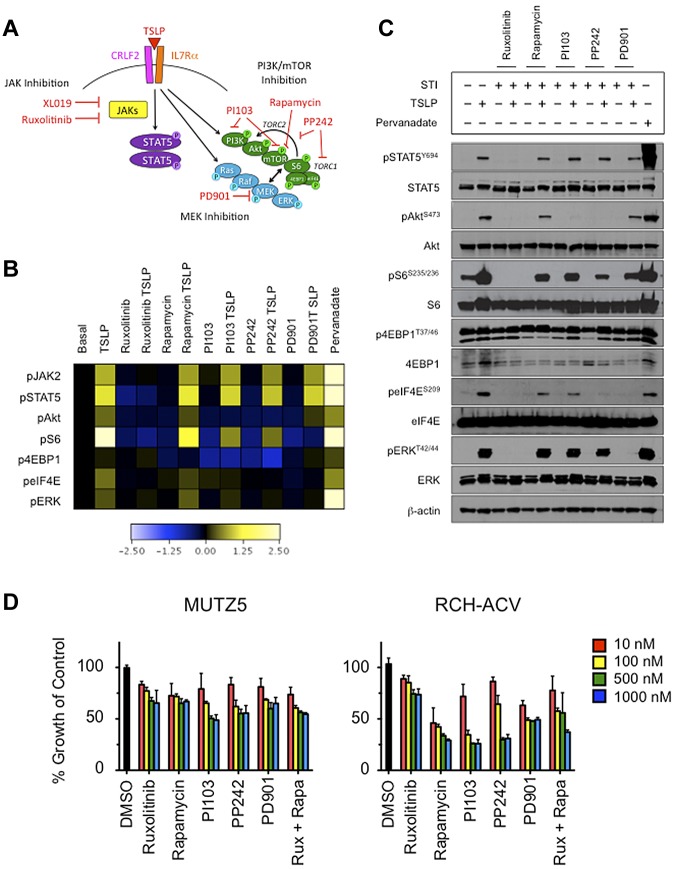
Figure 3.
In vitro stimulation of CRLF2r ALL with TSLP-induced phosphorylation of STAT5 and PI3K pathway proteins and, to a lesser extent, ERK. Phosphorylation of signaling proteins was abrogated by specific STIs. (A) Hypothetical schema of aberrant signal transduction mediated by the TSLPR heterodimer in CRLF2r ALL and strategies for targeting the perturbed signaling nodes with STIs. (B) Phosphoflow cytometry analysis of fixed and permeabilized MUTZ5 cells demonstrated increased phosphorylation of JAK2, STAT5, ERK, Akt, S6, and eIF4E after stimulation with TSLP. Inhibition of basal and/or TSLP-induced phosphorylation of relevant proteins was observed with specific STIs. (C) Western blotting analysis of MUTZ5. Cells demonstrated constitutive phosphorylation of S6 and 4EBP1. TSLP-induced phosphorylation of signal transduction proteins and abrogation of phosphorylation by specific STIs was generally concordant with phosphoflow cytometry results in panel A, although more pronounced TSLP-induced pERK was observed by Western blotting. (D) Cytotoxicity assays of MUTZ5 and RCH-ACV cell lines treated with STIs. Cells were treated with the specified STIs for 72 hours before performing MTT assays. Ruxolitinib induced moderate dose-dependent cytotoxicity in MUTZ5. The PI3K/mTOR STIs induced dose-dependent cytotoxicity in both cell lines. Data are normalized to DMSO controls (black). Red indicates 10 nM; yellow, 100 nM; green, 500 nM; blue, 1000 nM of each STI. Rux indicates ruxolitinib, and Rapa is rapamycin.