Abstract
Objective
Although the Malawian government recommends HIV-exposed infants receive early infant diagnosis of HIV (EID) at “under-five” pediatric clinics (U5Cs), most never enroll. Therefore, we evaluated the integration of EID testing into an immunization clinic (IC) compared to the current standard of EID testing at an U5C.
Design
Prospective observational study.
Methods
Using routine provider initiated HIV testing and counseling (PITC) registers, we prospectively studied 1757 children offered PITC at a government IC and U5C. Infants tested by HIV DNA polymerase chain reaction (PCR) were followed until PCR result disclosure or defaulting.
Results
We sampled 877 and 880 consecutive PITC recipients at U5C and IC, respectively. Overall, a 7 fold greater proportion received PITC at IC (84.2% vs 11.4%, p<0.001). PITC recipients at IC were more than 14 months younger (2.6 vs 17.0, p<0.001), with greater proportions HIV-exposed (17.6% vs 5.3%, p<0.001) and PCR eligible (7.9% vs 3.5%, p<0.001).
A higher percentage of IC infants accepted PCR testing (100.0% vs 90.3%, p=0.03). Additionally, IC PCR recipients were 2.5 months younger (3.1 vs 5.6, p<0.001) with 4 times less testing PCR positive (7.1% vs 32.1%, p<0.001). Importantly, a more than 3 fold greater proportion of HIV-exposed infants at IC returned for their PCR result and enrolled into care (78.6% vs 25.0%, p<0.001).
Conclusions
Compared to an U5C, integrating EID testing into an IC is more acceptable, more feasible, enrolls more infants into EID at younger ages, and would likely strengthen Malawi's EID services if expanded.
Keywords: Africa, pediatrics, prevention of mother-to-child transmission, early infant diagnosis of HIV, HIV DNA PCR, provider initiated HIV testing and counseling
INTRODUCTION
Malawi, a southern African country with a human immunodeficiency virus (HIV) prevalence of 11%1, implemented early infant diagnosis of HIV (EID) using DNA polymerase chain reaction (PCR) in 2007 to improve infant HIV care1. Unfortunately, the Malawi EID program has underperformed partly due to inadequately coordinated antenatal and post-natal HIV services2,3. As a result, even though HIV-exposed infants are recommended to enroll into EID at “under-five” general pediatric clinics (U5Cs), many instead access EID late or not at all2,3.
One alternative to the current standard of care is to integrate EID into immunization clinics (ICs), which are accessed by more than 90% of all Malawian children4. In Malawi U5Cs offer care for sick children, EID, and ICs. Although ICs occur at U5Cs, they remain distinct from EID. Instead the majority of EID testing at U5Cs takes place only when infants are brought for PCR especially or if they are sick2,3. IC patients, on the other hand, may be more ideal EID candidates since they are usually infants without acute illnesses. Therefore, we evaluated the acceptability, feasibility, and outcomes of EID integrated into an IC compared to the current standard of EID at an U5C.
MATERIALS AND METHODS
Study Setting
The IC at Bwaila Hospital and the U5C at Kamuzu Central Hospital are busy government clinics serving Lilongwe's population of 750,000 people4. While both pediatric clinics receive referrals, the vast majority receiving care at both sites are self-referred. A “pilot” provider initiated HIV testing and counseling (PITC) program at the IC began in January 2011, while at the U5C PITC services have been offered since June 2010. Each clinic's PITC service operated Monday to Friday with similar staffing levels and utilized volunteer patient escorts as described previously5. Specifically, one HIV counselor and one patient escort staffed one private HIV testing room at the IC, and two HIV counselors and one patient escort staffed the two private HIV testing rooms at the U5C. Patient escorts are parents of HIV-infected children responsible for HIV testing advocacy, assisting the counselor with administrative duties, and accompanying caregivers of HIV-affected infants from the testing room to the HIV clinic.
At both sites HIV counselors delivered group counseling to caregivers, who were predominantly mothers. At the IC each caregiver met with the HIV counselor after the child received the vaccination and was weighed. At the U5C, clinicians and nurses would refer patients for HIV testing based upon clinical suspicion while PITC staff recruited patients regardless of their presentation. In the testing rooms at both clinics, the counselor obtained verbal consent for HIV testing of the infant from the responsible caregiver in compliance with Malawi HIV testing guidelines6. Accordingly, HIV testing was offered if the mother's last HIV test was more than three months before that date, she had never been tested or documentation of her test history was unavailable, or a clinical indication warranted a repeat test. HIV testing information was recorded on a confidential but non-anonymous PITC register as outlined previously5. After disclosure of the HIV test result, all newly identified HIV-infected or HIV-exposed children were prescribed cotrimoxazole prophylaxis (if older than 6 weeks) and were accompanied by a patient escort to a nearby HIV clinic.
Study Design
Using the PITC registers, we prospectively sampled a total of 1757 children offered HIV testing at the IC and U5C, respectively, beginning February 2011. Patients were followed until disclosure of the PCR result, which was scheduled four weeks after testing according to EID protocol2. For the purposes of this study, an additional two weeks were allowed for PCR result disclosure if the patient missed their appointment, at which time they were considered a defaulter.
De-identified PITC data was entered into an electronic database for analysis. Ethical approval was obtained from the Malawi National Health Sciences Research Committee, University of North Carolina-Chapel Hill School of Medicine, and Baylor College of Medicine. Consent from individual participants was not required since this study was within the routine operations of the Malawi Ministry of Health PITC program and involved no risk or direct contact with subjects.
Definitions
Eligible for PCR testing
Children were eligible for PCR if they were younger than 12 months of age, their mother was HIV-infected, and they were not already enrolled in HIV care elsewhere with a previous or pending PCR.
HIV-exposed
HIV-exposed children were defined as children younger than 12 months of age and either breastfeeding from an HIV-infected mother or not breastfeeding but without a definitively negative PCR result.
HIV-infection
HIV-infected children were older than 12 months of age and tested HIV-antibody positive, or had a documented positive PCR irrespective of their age.
Statistical Methods
The sample was based upon our hypothesis that the proportion of PCRs would represent 3.9% of HIV tests at the U5C and that this would increase to 7.0% at the IC. Associations between covariates were tested using Chi-square, Fisher's exact, and Wilcoxon signed rank tests; alpha was 0.05. Analysis was conducted using SAS (SAS institute, Cary, NC, USA).
RESULTS
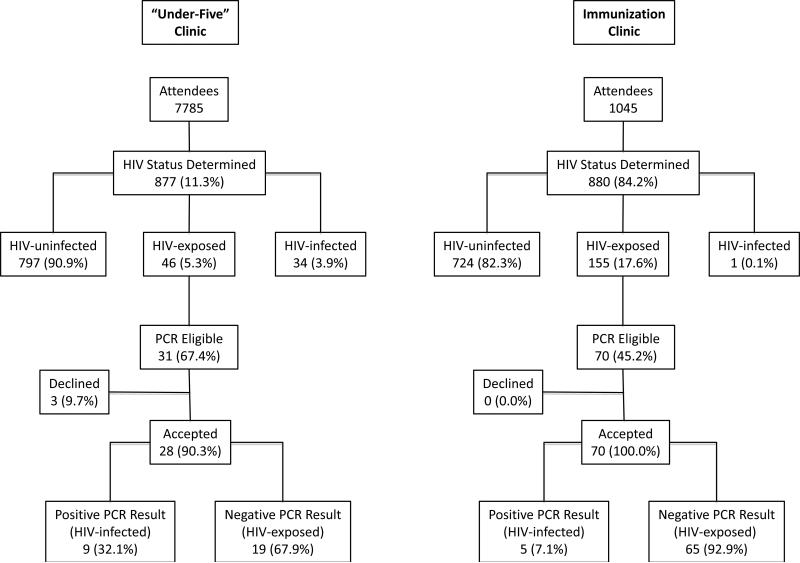
We sampled 877 and 880 successive PITC recipients at the U5C and IC, respectively (Figure 1). The uptake of PITC at the IC and the U5C was compared, and we discovered a 7-times greater proportion of children accessed PITC at the IC (84.2% vs 11.4%, p<0.001). Overall patient characteristics including age, gender, initial HIV status, and PCR eligibility were evaluated. No difference in gender was identified between infants at both clinics. However, children that received PITC at the IC were found to be considerably younger (2.6 vs 17.0 months, p<0.001), with much higher percentages identified as HIV-exposed (17.6% vs 5.3%, p<0.001) and eligible for PCR testing (7.9% vs 3.5%, p<0.001).
Figure 1.
Study population diagram. HIV indicates human immunodeficiency virus; PCR, polymerase chain reaction.
This study also assessed the acceptability of EID at both sites and investigated the characteristics of patients that received a PCR. We found that a higher percentage of infants at the IC accepted PCR testing (100.0% vs 90.3%, p=0.03). Additionally, PCR recipients at the IC were 2.5 months younger (3.1 vs 5.6, p<0.001) with higher proportions having received prevention of mother-to-child HIV transmission (PMTCT) interventions (85.7% vs 40.0%, p<0.001) and 4 times less PCR positive (7.1% vs 32.1%, p<0.001). We also found that a more than 3-times higher proportion of HIV-exposed infants at the IC returned to the counselor after 4 weeks to receive their PCR result and then enrolled into care at the HIV clinic (78.6% vs 25.0%, p<0.001).
DISCUSSION
The Malawi EID program currently recommends that HIV-infected mothers bring their infants at 6 weeks of age to an under-five clinic for PCR and evaluation even if the child is well. In reality, almost half of HIV-exposed infants are not enrolled into EID at these clinics and never receive a PCR at all or only after acquiring an illness, which is often HIV-related2,3. Of those infants that receive a PCR at under-five clinics, more than 2/3 fail to return for their PCR result and do not enroll into EID care2,3. Therefore, alternative EID entry points require evaluation. The most appealing site for integrating EID programming would be one that is already routinely used by children and could absorb an additional service with minimal disruption. For these reasons, we compared a pilot program that integrated EID into an IC to the current standard of EID at an U5C. Our results clearly demonstrate that IC EID testing is more feasible for providers, more acceptable for caregivers of PCR eligible infants, and enrolls and retains greater numbers of younger patients into EID.
Ideally, EID programs should identify all HIV-exposed infants at the youngest age possible, prior to HIV-infection, so infants can maximally benefit from early access to HIV PCR testing, maternal or infant PMTCT interventions, breastfeeding counseling, and cotrimoxazole prophylaxis. Similarly, early identification of HIV-infected infants is critical since the greatest survival benefit from ART is realized if it is urgently initiated early in life2,7. Targeting patients that maximally benefit from these interventions is the best use of costly EID resources in any situation, but particularly in settings with severe constraints like Malawi. Opt-out HIV antibody testing of mothers or infants could achieve these goals8. Importantly, we found that PCR recipients at this IC were younger by 2.5 months with far less testing PCR positive. Furthermore, HIV-infected infants identified at IC were also nearly 4 months younger than those at U5C (data not shown). These data strongly support the notion that PITC at ICs, compared to testing at U5Cs, is a better use of resources by targeting patients that can benefit the most from EID services.
Given that resources are limited, any further scale-up of EID programming must consider whether the clinical setting best facilitates the delivery of services to the greatest numbers of patients. For example, although the two PITC sites utilized similar staffing levels, we found the proportion of patients offered HIV testing at the IC to be more than seven-times higher than at the U5C. This striking disparity may be partially explained by the differing characteristics of the sites with respect to patient acuity and volume. More specifically, most patients attend the U5C due to an acute illness and often require multiple services including clinical assessment, laboratories, radiology, and pharmacologic interventions. On the other hand, most IC attendees are healthy and require only anthropomorphic measurements and vaccinations. HIV testing at the U5C is made even more difficult by higher patient volumes (171 vs 52 patients/day at the IC). Overall these findings suggest that ICs may be a more suitable clinical setting for the most efficient delivery of EID services to the greatest number of patients.
In addition to targeting the most eligible patient population and selecting the most suitable clinical setting, client acceptance is another key factor for measuring the success of integrating EID testing into an existing service. Using PCR acceptance rates and the retention of PCR recipients as measures of acceptability, this study also indicates that caregivers of PCR eligible infants found PITC at an IC more acceptable. No patients declined PCR testing at the IC, and even more importantly, more than three times more PCR recipients returned for their PCR result and were enrolled into the EID program. Notably, these were infants who otherwise may not have accessed PCR without the availability of integrated IC EID services. Our results strengthen findings from a South African study that reported similar client acceptance rates of EID testing at ICs9, and taken together may suggest that the integration of EID services and ICs would be acceptable throughout the southern African region.
One limitation is this study compares just two urban clinics. While a multisite comparison including rural clinics is an important next step, we were unaware of other similarly integrated IC and EID services in Malawi at the time of this study.
In conclusion, we recommend the integration of opt-out HIV testing and counseling at ICs for all eligible mothers and infants. Scaling up EID testing at ICs is likely to strengthen EID services in Malawi.
Table 1.
Characteristics of Early Infant Diagnosis of HIV Testing at an “Under-Five” General Pediatric Clinic and an Immunization Clinic
| “Under-Five” Clinic (N=877) | Immunization Clinic (N=880) | P-value | ||
|---|---|---|---|---|
| Characteristics of Study Population | Age in Months, Median (IQR) | 17.0 (9.1 – 37.0) | 2.6 (1.3 – 7.0) | <0.001 |
| Females, n (%) | 440 (50.6) | 430 (49.1) | 0.53 | |
| Child's Enrollment HIV status | <0.001 | |||
| HIV-Uninfected, n (%) | 797 (90.9) | 724 (82.3) | ||
| HIV-Exposed, n (%) | 46 (5.3) | 155 (17.6) | ||
| HIV-Infected, n (%) | 34 (3.8) | 1 (0.1) | ||
| PCR test eligible, n (%) | 31 (3.5) | 70 (7.9) | <0.001 | |
| Characteristics of HIV PCR Recipients | PCR test accepted, n/N (%) | 28/31 (90.3) | 70/70 (100.0) | 0.03 |
| Age in Months, Median (IQR) | 5.6 (3.3 – 9.1_) | 3.1 (2.3 – 5.2) | <0.001 | |
| Females, n/N (%) | 16/28 (57.1) | 33/70 (47.1) | 0.37 | |
| PCR Positive, n/N (%) | 9/28 (32.1) | 5/70 (7.1) | <0.001 | |
| Breast feeding, n/N (%) | 25/28 (89.3) | 69/70 (98.6) | 0.06 | |
| Child received PMTCT, n/N1 | 6/15 (40.0) | 60/70 (85.7) | <0.001 | |
| Mother received PMTCT, n/N (%)2 | 7/14 (50.0) | 62/69 (89.9) | <0.001 | |
| Received PCR result, n/N (%) | 7/28 (25.0) | 55/70 (78.6) | <0.001 |
HIV indicates human immunodeficiency virus; IQR, interquartile range; PCR, polymerase chain reaction; PMTCT, prevention of mother-to-child transmission.
13 children from “Under-Five” Clinic did not have PMTCT information documented.
14 mothers from “Under-Five” Clinic and 1 mother from Immunization Clinic did not have PMTCT information documented.
Acknowledgements
Conceived and designed the experiments: EDM, CSC, DO, MCH. Performed the experiments: EDM. Analyzed the data: EDM, DCJ. Wrote the manuscript: EDM, DCJ. Critical review of manuscript: CSC, LDS, PNK, DO, IH, CVDH, MCH. This study was supported in part by the National Institutes of Health (R24 TW007988) through the Fogarty International Center and International Clinical Research Fellows Program at Vanderbilt University and the University of North Carolina Center for AIDS Research (5 P30-AI50410). We are grateful to Dr. Geoffrey A. Preidis, PhD for his contributions to the design of the study. Our gratitude is extended to the HIV counselors and patient escorts who provided HIV counseling services to study participants at Bwaila Hospital and Kamuzu Central Hospital.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.UNAIDS/WHO . AIDS epidemic update 2009. World Health Organization; Geneva: 2009. [Google Scholar]
- 2.Braun M, Kabue MM, McCollum ED, et al. Inadequate Coordination of Maternal and Infant HIV Services Detrimentally Affects Early Infant Diagnosis Outcomes in Lilongwe, Malawi. J Acquir Immune Defic Syndr. 2011;56:e122–e128. doi: 10.1097/QAI.0b013e31820a7f2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Lettow M, Bedell R, Landes M, et al. Uptake and outcomes of a prevention-of-mother-to-child transmission (PMTCT) program in Zomba district, Malawi. BMC Public Health. 2011;11:426. doi: 10.1186/1471-2458-11-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.2010 Malawi Demographic and Health Survey. ICF Macro Office; Zomba, Malawi: 2011. [Google Scholar]
- 5.McCollum ED, Preidis GA, Kabue MM, et al. Task Shifting Routine Inpatient Pediatric HIV Testing Improves Program Outcomes in Urban Malawi: A Retrospective Observational Study. PLos ONE. 2010;5:3. doi: 10.1371/journal.pone.0009626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ministry of Health Malawi: HIV Unit Guidelines for Paediatric HIV Testing and Counseling. 2008 [Google Scholar]
- 7.Violari A, Cotton MF, Gibb DM, et al. Early Antiretroviral Therapy and Mortality among HIV-Infected Infants. N Engl J Med. 2008;21:2233–2244. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kellerman S, Essajee S. HIV Testing for Children in Resource-Limited Settings: What Are We Waiting For? PLoS Med. 2010;7:7. doi: 10.1371/journal.pmed.1000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rollins N, Mzolo S, Moodley T, et al. Universal HIV testing of infants at immunization clinics: an acceptable and feasible approach for early infant diagnosis in high HIV prevalence settings. AIDS. 2009;14:1851–1857. doi: 10.1097/QAD.0b013e32832d84fd. [DOI] [PubMed] [Google Scholar]