Abstract
We present the development and validation of a theory-derived scale measuring patients' behavioral intention to adhere to HIV care. Adherence to HIV care includes attendance at appointments and adherence to highly active antiretroviral therapy (HAART) regimens. These two components have been independently associated with long-term HIV outcomes. Items were chosen to reflect behavioral intention as defined by the Health Action Process Approach to health-seeking behavior. Items reflecting self-reported HIV knowledge were also included after expert panel review. The study took place from October 2009 to April 2010 at two HIV clinics in Houston, Texas. Participants were 287 adults with HIV/AIDS (10.1% female, mean age 50.8); 56.5% were African-American and 17% were Hispanic. Of the total, 87.1% were on HAART at enrollment. Factor analysis of survey items resulted in the retention of two domains, knowledge and intention, based on scree plot analysis of eigenvalues. Questions with factor loadings >0.4 were retained, yielding 4 knowledge questions and 10 intention questions. The survey had good internal consistency for knowledge (Cronbach's α=0.83) and for intention (Cronbach's α=0.81). In multivariate analysis, intention was associated with HIV viral suppression, defined as HIV-1 viral load <400 RNA copies/mL, (odds ratio [OR]=1.75, 95% .confidence interval [CI]=1.00–3.07). Knowledge was also associated with HIV suppression (OR=1.55, 95%, CI=1.09–2.12). The resulting study describes the development and preliminary validation of an HIV treatment-seeking intention measure. Additional studies are needed to validate this instrument in other populations.
Introduction
Advances in medication therapy for human immunodeficiency virus (HIV) have resulted in significant improvements in HIV control. The introduction of highly active antiretroviral therapy (HAART) in 1996 marked a transformation in patient outcomes.1 Patient adherence to HIV care is essential for realization of the full benefits of HAART.2,3 Adherence to HIV care is a concept that includes attendance at appointments and adherence to HAART regimens.4,5 These two components have been shown to have independent associations with long-term healthcare outcomes. More than 10% of patients taking HAART report missing one or more doses of medications per day, and >33% report missing doses over a 2- to 4-week period.2 In the past, studies evaluating adherence to protease inhibitor therapy concluded that adherence of ≥95% was required to achieve viral load suppression.6 Newer antiretroviral regimens, including ritonavir-boosted protease inhibitors and non-nucleoside reverse transcriptase inhibitor based regimens, have been shown to be effective with somewhat lower levels of adherence.7
Even with these more forgiving regimens, retention in care is still critical to obtain the full benefits of HAART.8 Studies have shown that up to 40% of HIV patients may fall short of recommendations for retention in HIV care.3 Poor retention in HIV care has been associated with delayed receipt of HAART, higher viral loads, lower CD4 counts, more hospital admissions, more emergency department visits, and shorter survival.1,8,9 Numerous factors can affect HIV care adherence. Linkage and retention in care have been shown to be influenced by factors such as age and race, the amount of time since diagnosis, the use of injection drugs, and the source of referral to HIV care.10–12 Other factors that have been explored for their association with HIV care adherence include psychosocial problems, psychiatric comorbidities, the physician–patient relationship, physician communication style, patient knowledge about HIV, HAART regimen nature and side effects, and disease characteristics such as the neurocognitive aspects of HIV/AIDS.13–19 These studies add considerably to our knowledge of the empirical factors that moderate HIV-related behaviors. However, there is still a need for more theory-driven studies that will elucidate treatment adherence patterns among HIV patients.
Literature from the behavioral sciences offers several validated models to predict health-seeking behaviors and to identify motivational factors that can serve as targets for intervention. Behaviors as diverse as smoking cessation, diet, exercise, cancer screening, sunscreen use, and blood donation have been examined with such models.20–29 The best-known models of health-seeking behaviors are the theory of planned behavior and its predecessor, the theory of reasoned action, as well as the health action process approach (HAPA).30,31 HAPA has previously been used to predict diet and exercise behavior.30–32 We believe it is highly applicable to HIV treatment-adherence behaviors.
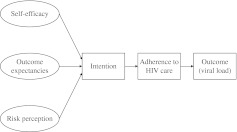
Within the HAPA model, the concept of behavioral intention—defined as an individual's commitment to achieve a goal or to perform an action—is believed to have a central influence over that individual's resulting behavior.30,31 During the past 30 years, a wealth of social science evidence has confirmed the predictive value of behavioral intention.33 For example, in studies of hypertension control, behavioral intention has been associated with medication adherence.34 HAPA asserts that behavioral intention consists of three subdomains: self-efficacy, risk perception, and outcome expectancy (Fig. 1).31 Self-efficacy reflects an individual's confidence that he or she can accomplish a task even in the face of potential challenges. Risk perception captures an individual's understanding of a health threat. Outcome expectancies are beliefs about the likely positive and negative results of an action.
FIG. 1.
Application of the health action process approach to adherence to HIV care.
There are a growing number of studies that evaluate patients' self-efficacy and intention to adhere to HAART.35–38 However, no validated scale of patients' intention to adhere to HIV care has yet been published. A number of guidelines suggesting how HAART should be administered, including the United States Department of Health and Human Services guidelines and the British HIV guidelines, agree that therapy should be started after an assessment of readiness, a concept related to intention.39 A recent review of the literature on readiness found no existing readiness measure that demonstrated clinical utility as a predictor of adherence.39 Our objective was to develop an instrument that would be useful in measuring patients' self-assessed intention to adhere to care and to validate the scale against measures of HIV control. The purpose of this article is to describe the development and validation of an HIV care adherence intention measure, called the HIV Intention Measure (HIV-IM) (Appendix 1).
Methods
Settings
Participants were recruited from HIV clinics at the Michael E. DeBakey VA Medical Center (VAMC) and the Thomas Street Health Center (TSC) in Houston, Texas. Both clinics exclusively serve adult patients with HIV. The VAMC provides care to veterans from urban, suburban, and rural areas of Southeast Texas; ∼850 HIV-infected subjects are enrolled in the HIV clinic; risk factors for HIV infection included men who have sex with men in 31% and intravenous drug use in 7%. The TSC is a freestanding HIV/AIDS treatment facility operated by the Harris County Hospital District that provides comprehensive medical services to residents of Harris County and surrounding counties who are diagnosed with HIV/AIDS, regardless of ability to pay. The TSC is among the largest HIV clinics in the United States, serving >4500 patients in 2009. Of TSC patients, 29% identify as men who have sex with men whereas 7% report using intravenous drugs.
Patients were recruited between October 2009 and April 2010 from the waiting rooms at both clinics. There were no exclusion criteria. Patients were approached by study staff after checking in for their appointments and while waiting to see their physicians. Oral consent was obtained and patients were given the survey. On average, participants spent 15–30 min completing the survey. Surveys were filled out by 311 patients, of whom 15 were excluded from analysis because of incomplete responses, and 9 were excluded because they had only recently started treatment. Results from the remaining 287 questionnaires were included in factor analysis. Of these, 271 and 16 participants completed the English and Spanish version of the survey, respectively. Patients' charts were reviewed to determine the HIV viral load results measured closest in time prior to the visit in which the survey was taken.
Development and validation of patient questionnaires
All study participants were asked to complete a 53-question survey that included seventeen items about self-assessed HIV knowledge and intention to remain in care. Each item had six possible responses with a score of one to six along a Likert scale (see Appendix 1). The content of the survey items was informed by HAPA and its definition of behavioral intention.
Content and face validity
Content validity implies that a scale adequately surveys the domain being assessed, in this case self-assessed intention to adhere to HIV care. We incorporated five questions adapted from a previously validated scale that measured behavioral intention in the setting of hypertension treatment.34 Twelve new questions were designed in order to ensure that self-efficacy, risk perception, and outcome expectancy as they related to treatment adherence were each represented. Four infectious disease experts reviewed the initial 53 question survey questions for thoroughness and appropriateness. These coauthors (SG, TG, MR, BT) agreed that it was worthwhile to explore the relationship between self-assessed HIV care intention, self-assessed HIV knowledge, and adherence to treatment. We agreed with their recommendations, hypothesizing that patients' assessment of their perception of their HIV knowledge might be related to their intention to adhere to treatment. Four items pertaining to self-reported HIV knowledge were among the questions included in this survey.
We assessed face validity using cognitive testing methodology.40 Study staff members held cognitive interviews with patients both in English and Spanish for the purpose of clarifying and revising questions. During the cognitive interviews, survey questions were read aloud to patients similar to those who later completed questionnaires. These patients were asked to describe what they thought each question was asking. A total of nine patients participated in cognitive interviews (seven at the VAMC and two at TSC). These interviews confirmed contextual understanding of each of the items by respondents, without additional changes to any survey items. The questions were also translated into Spanish by two native Spanish speakers. A cognitive interview was conducted at TSC with a Spanish-speaking patient, with similar results.
Construct validity
is the extent to which an instrument measures the constructs it was designed to measure, in this case behavioral intention for seeking HIV treatment. Exploratory factor analysis, using an orthogonal rotation method, was performed on our 287 completed questionnaires to uncover the underlying domains of the 17 items related to self-assessed knowledge and intention to remain in care.40,41 Factor analysis is a statistical technique that identifies underlying domains in a large number of questions.41
The scree test was used initially to identify factors with large eigenvalues that were assumed meaningful and were subsequently retained for rotation.41 In addition, factors accounting for ≥10% of the variance in the data were retained. To determine the number of factors, we identified factors composed of at least three items with significant loadings on each retained factor. Factor loadings of 0.40 from the rotated factor pattern were considered important. We required that the items making up the factors had high factor loadings on only one factor and near-zero loadings for the other factors.41 After determining which factors would be retained, the meanings of the retained factors were interpreted. We then measured internal consistency, using Cronbach's coefficient α, to determine the variance between items in a scale and the internal consistency of the full scale.40
Predictive validity
We tested predictive validity by determining the relationship of our retained factors with whether the patient had achieved HIV viral suppression to a viral load of <400 copies/mL of blood (defined as “HIV control”). Poor adherence has been shown to be an independent predictor of virologic failure.6,42 The relationship between adherence to HIV care and HIV control is well established, providing an important clinical and methodological standard for establishing our intention measure validity.3,9
Predictive validity was evaluated using logistic regression models of HIV control (dependent variable) with each of our retained factors (independent variables), adjusting for the following study covariates: age, gender, ethnicity (Hispanic, non-Hispanic), race (white, black, Asian, other), education (some secondary education, high school graduate, any college), length of HIV diagnosis, and site of care (VA or TSC). Odds ratios (OR) and 95% confidence intervals (CI) for the primary independent variables were calculated.
Results
Study population
Baseline characteristics of the study participants are described in Table 1 Differences among these characteristics were stratified by clinic location because of significant differences in the populations served by both sites. VAMC patients were older than TSC patients and were more likely to be male, to have graduated from high school, to have attended some college, and to be African-American. VAMC patients were less likely to be Hispanic. More patients were on HAART at the VAMC (90.3%) than at TSC (77.5%). Viral load was lower among VAMC patients (mean 2.2 log10 copies/mL) than among TSC patients (mean 2.6 log10 copies/mL).
Table 1.
Characteristics and Demographics of the Study Population
| Patient characteristics | Total (n=287) |
|---|---|
| Age, years, mean (SD) | 50.8 (9.9) |
| Gender | |
| Female, n (%) | 29 (10.1) |
| Male, n (%) | 258 (89.9) |
| Race | |
| Black, n (%) | 162 (56.5) |
| White, n (%) | 112 (39.0) |
| Other, n (%) | 13 (4.5) |
| Ethnicity | |
| Non-Hispanic, n (%) | 234 (83.0) |
| Hispanic, n (%) | 48 (17.0) |
| Education | |
| <High school, n (%) | 33 (11.6) |
| High school/GED, n (%) | 91 (31.9) |
| Some college or more, n (%) | 161 (56.5) |
| CD4 count, cells/mm3 | |
| Mean (SD) | 468.3 (286.8) |
| Median (range) | 427.5 (4.0–1491.0) |
| HIV viral load, copies/mL, log10 | |
| Mean (SD) | 2.3 (1.2) |
| Median (range) | 1.7 (1.7–6.9) |
| Patients on HAART, n (%) | 250 (87.1) |
| Patients with HIV viral load <400 copies/mL, n (%) | 222 (80.1) |
| Time since HIV diagnosis | |
| <1 yr, n (%) | 14 (4.9) |
| 1–10 yrs, n (%) | 100 (34.8) |
| >10 yrs, n (%) | 173 (60.3) |
SD, standard deviation; GED, general equivalency diploma; HIV, human immunodeficiency virus; HAART, highly active antiretroviral therapy.
Results of exploratory factor analysis
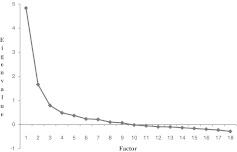
The scree plot was used initially to establish the number of distinct factors (see Fig. 2). The scree plot showed a break between factors 2 and 3, indicating that the first two factors, both with eigenvalues >1, were the most meaningful. The first factor accounted for 64% of the variance, and the second factor accounted for 22% of the variance. The third factor accounted only for 10%, and later factors accounted for increasingly small percentages of variance. Therefore, our scree test confirmed a two-factor solution. Next, we determined the rotated factor loadings as given in Table 2. Items with factor loadings of <0.40 were removed from the scale. No items were removed from factor 2. The final step in the exploratory factor analysis included interpretation of the selected factors. Factor 1 made a large and unique contribution to the variance of the 10 items that were retained in the measure, that is, items 5, 6, 7, 8, 9, 11, 12, 14, 15, and 17. Because these items relate to risk perception, outcome expectancy, and self-efficacy, factor 1 was labeled “intention to adhere to HIV treatment.” Factor 2 made a unique and noticeable contribution to the variance of items 1, 2, 3, and 4. Because these items were designed to assess knowledge, factor 2 was labeled “HIV knowledge.”
FIG. 2.
A scree plot of eigenvalues after exploratory factor analysis. Factors are shown in order of decreasing eigenvalues. Factors 1 and 2, with eigenvalues >1.0 and appearing in the vertical portion of the graph, were retained and interpreted, whereas factors with eigenvalues <1.0 and appearing in the horizontal portion of the graph were disregarded.
Table 2.
Exploratory Factor Analysis: Factor Loadings by Item
| Items | Factor 1 (intention) | Factor 2 (knowledge) |
|---|---|---|
| 1. I know a lot about living with HIV infection. | 0.39 | 0.70a |
| 2. I know a lot about how HIV is spread from one person to another. | 0.31 | 0.62a |
| 3. I know a lot about medication to treat HIV infection. | 0.26 | 0.85a |
| 4. I know a lot about the side-effects of medications used to treat HIV infection. | 0.18 | 0.78a |
| 5. Coming regularly to my HIV clinic appointments is good for my health. | 0.72a | 0.39 |
| 6. My treatment plan for HIV will make a big difference in keeping my HIV infection under control. | 0.81a | 0.35 |
| 7. HIV medications help to control HIV disease. | 0.63a | 0.23 |
| 8. If HIV medications are prescribed, it is important to take the medications everyday to control HIV infections. | 0.69a | 0.23 |
| 9. Not taking HIV medications every day affects how well the HIV treatment works. | 0.49a | 0.18 |
| 10. The HIV virus can become resistant to the medications. | 0.22 | 0.23 |
| 11. An HIV patient who is feeling well can safely stop taking HIV medications. | 0.50a | 0.14 |
| 12. An HIV infected person who follows recommended care for HIV can expect to live long. | 0.44a | 0.10 |
| 13. An HIV infected person on HIV medications can spread the HIV virus by having sex without a condom. | 0.32 | 0.18 |
| 14. There is a lot I can do to control my HIV infection. | 0.63a | 0.30 |
| 15. What I do can determine whether my HIV infection gets better or worse. | 0.66a | 0.34 |
| 16. Nothing I do will affect my HIV infection | 0.40 | 0.9 |
| 17. My actions will have no effect on the outcome of my HIV infection. | 0.50a | 0.25 |
| Eigenvalue | 4.83 | 1.66 |
Values >0.4.
Internal consistency
The scale showed high internal consistency for items within each of the two specified factors. Cronbach's α was 0.81 for factor 1 (intention) and 0.83 for factor 2 (knowledge). These results establish an appropriate level of internal consistency between both factors.
Predictive validity
Because knowledge and intention were found to be highly correlated (p<0.001), the two factors were modeled in separate logistic models of HIV control. Model 1 assessed the relationship between intention and HIV control. Model 2 assessed the relationship between HIV knowledge and HIV control. After adjusting for relevant sociodemographic and clinical covariates, the intention factor was significantly associated with HIV control (OR=1.75, 95% CI=1.00–3.07). This odds ratio indicates that the odds of a patient's HIV being controlled increased by 1.75 times for each increase of 1 unit in the value of the intention factor. Similarly, the knowledge factor was also independently associated with HIV control after controlling for the same covariates (OR=1.55, 95% CI=1.09–2.12). Therefore, a patient's odds of having their HIV controlled increased by 1.55 times for each unit increase in their self-reported knowledge about HIV.
Discussion
The current study describes the development and preliminary validation of the HIV-Intention Measure (HIV-IM). This is a novel patient-reported scale of respondents' intention to adhere to HIV care. Development of the HIV-IM scale involved identifying individual items based on behavioral theory, eliciting expert opinions regarding patient adherence to HIV care, and confirming item understanding using cognitive interviews. Exploratory factor analysis validated the factor structure into two distinct factors, consisting of a self-reported HIV knowledge factor and a behavioral intention factor. Items within these two distinct factors (intention and knowledge) were internally consistent. Multivariate logistic regression models confirmed the predictive validity of both the intention and knowledge factors through significant associations with HIV control.
As expected from behavioral theory, HIV self-assessed knowledge and intention to adhere to HIV care were distinct factors. However, the predictive validity of each factor could not be analyzed independently of the other given the strong collinearity of both factors. The current study, which measured self-assessed knowledge, found that it was associated with HIV control. This would seem to be consistent with studies that have shown that HIV knowledge-obtaining behaviors, such as Internet health information seeking, are predictive of medication adherence.43 We must emphasize that HIV knowledge as measured in our survey was self-assessed, with questions worded as “I know a lot about” the knowledge concept being tested. We did not attempt to assess actual knowledge through a factual quiz but instead focused on the patient's sense of mastery of the knowledge.
The finding that intention to adhere to HIV care is associated with HIV control is especially novel, as prior measures have focused on the intention to adhere to HIV medications.35,36,38 Adherence to HIV care is a more comprehensive concept than adherence to HAART, and encompasses medication adherence as one of its elements.4,5 The validation analyses conducted in this study suggest that within the context of adherence to HIV care, intention is a clinically important variable. Furthermore, the factor analysis found that intention was a single factor rather than a three or four component construct. However, it is possible that if we had increased the number of discrete questions assessing the subdomains of intention described in the HAPA model, namely risk perception, self-efficacy, and outcome expectancy, these concepts might have emerged as separate factors. However, a longer survey would have increased participants' response burden while decreasing acceptability. Previous studies have found that there is an association between patients' belief in the efficacy of HAART and adherence.44,45 Future validation studies with different HIV populations and with more discrete questions focused on various subdomains are needed to confirm our findings.
Strengths of our scale development include that it was developed in two distinct types of HIV outpatient clinics enrolling diverse populations of HIV-positive patients. On the other hand, the HIV-IM scale was developed using a population of patients who were already in care and were already on therapy for HIV. Further studies are needed to evaluate treatment-seeking behaviors among HIV patients who are newly diagnosed or who are not currently engaged in care. Additional research can help elucidate whether intention at the time of HIV diagnosis correlates with subsequent treatment adherence and HIV control. Further study is also needed to determine if a one-time assessment or repeated assessments of intention to remain in HIV care will predict adherence to HIV care over time. However, our validated measure of intention is an important step forward. We envision future studies using this measure as a tool to facilitate development of public health interventions that foster better adherence to HIV care.
Supplementary Material
Acknowledgments
This article is the result of work supported with resources and use of facilities at the Houston VA Health Services Research and Development Center of Excellence (HFP90-020) at the Michael E. DeBakey VA Medical Center. Dr. Naik is also supported by a National Institute on Aging Career Development Award (K23AG027144-5) and by a Doris Duke Charitable Foundation Clinical Scientist Development Award. Dr. Trautner is supported by a VA Career Development Award from RR&D (B4623).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Horstmann E. Brown J. Islam F. Buck J. Agins B. Retaining HIV-infected patients in care: Where are we? Where do we go from here? Clin Infect Dis. 2010;50:752–761. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 2.Ickovics J. Meade C. Adherence to HAART among patients with HIV: breakthroughs and barriers. AIDS Care. 2002;14:309–318. doi: 10.1080/09540120220123685. [DOI] [PubMed] [Google Scholar]
- 3.Marks G. Gardner L. Craw J. Crepaz N. Entry and retention in medical care among HIV-diagnosed persons: a meta-analysis. AIDS. 2010;24:2665–2678. doi: 10.1097/QAD.0b013e32833f4b1b. [DOI] [PubMed] [Google Scholar]
- 4.Gardner E. McLees M. Steiner J. Del Rio C. Burman W. The spectrum of engagement in HIV care and its relevance to test-and-treat strageties for prevention of HIV infection. Clin Infect Dis. 2011;52:793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giordano T. Suarez–Almazor M. Grimes R. The population effectiveness of highly active antiretroviral therapy: are good drugs good enough? Curr HIV/AIDS Rep. 2005;2:177–183. doi: 10.1007/s11904-005-0013-7. [DOI] [PubMed] [Google Scholar]
- 6.Paterson D. Swindells S. Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients ith HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 7.Shuter J. Forgiveness of non-adherence to HIV-1 antiretroviral therapy. J Antimicrob Chemother. 2008;61:769–773. doi: 10.1093/jac/dkn020. [DOI] [PubMed] [Google Scholar]
- 8.Giordano T. Gifford A. White A. Retention in care: a challenge to survival with HIV infection. Clin Infect Dis. 2007;44:1493–1499. doi: 10.1086/516778. [DOI] [PubMed] [Google Scholar]
- 9.Mugavero M. Davila J. Nevin C. Giordano T. From access to engagement: measuring retention in outpatient HIV clinical care. AIDS Patient Care STDS. 2010;24:607–613. doi: 10.1089/apc.2010.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torian L. Wiewel E. Continuity of HIV-related medical care, New York City, 2005–2009: do patients who initiate care stay in care? AIDS Patient Care STDS. 2011;25:79–88. doi: 10.1089/apc.2010.0151. [DOI] [PubMed] [Google Scholar]
- 11.Pollini R. Blanco E. Crump C. Zuniga M. A community based study of barriers to HIV care initiation. AIDS Patient Care STDS. 2011;25:601–609. doi: 10.1089/apc.2010.0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hightow–Weidman L. Jones K. Wohl A, et al. Early linkage and retention in care: findings from the outreach, linkage, and retention in care initiative among young men of color who have sex with men. AIDS Patient Care STDS. 2011;25(Suppl 1):S31–S38. doi: 10.1089/apc.2011.9878. [DOI] [PubMed] [Google Scholar]
- 13.Boarts J. Sledjeski E. Bogart L. Delahanty D. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10:253–261. doi: 10.1007/s10461-006-9069-7. [DOI] [PubMed] [Google Scholar]
- 14.Ickovics J. Meisler A. Adherence in AIDS clinical trials: a framework for clinical research and clinical care. J Clin Epidemiol. 1997;50:385–391. doi: 10.1016/s0895-4356(97)00041-3. [DOI] [PubMed] [Google Scholar]
- 15.Kalichman S. Pope H. White D, et al. Association between health literacy and HIV treatment adherence: further evidence from objectively measured medication adherence. J Int Assoc Physicians AIDS Care. 2008;7:323. doi: 10.1177/1545109708328130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammed H. Kieltyka L. Richardson–Alston G, et al. Adherence to HAART among HIV-infected persons in rural Louisiana. AIDS Patient Care STDS. 2004;18:289–296. doi: 10.1089/108729104323076025. [DOI] [PubMed] [Google Scholar]
- 17.Schneider J. Kaplan S. Greenfield S. Li W. Wilson I. Better physician–patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med. 2004;19:1096–1103. doi: 10.1111/j.1525-1497.2004.30418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stone V. Jordan J. Tolson J. Miller R. Pilon T. Perspectives on adherence and simplicity for HIV-infected patients on antiretroviral thearpy: self-report of the relative importance of multiple attributes of highly active antiretroviral therapy (HAART) regimens in predicting adherence. J Acquir Immune Defic Syndr. 2004;36:808–816. doi: 10.1097/00126334-200407010-00007. [DOI] [PubMed] [Google Scholar]
- 19.Weiss L. French T. Finkelstein R. Waters M. Mukherjee R. Agins B. HIV-related knowledge and adherence to HAART. AIDS Care. 2003;15:673–679. doi: 10.1080/09540120310001595159. [DOI] [PubMed] [Google Scholar]
- 20.Armitage C. Conner M. Distinguishing perceptions of control from self-efficacy: predicting consumption of a low-fat diet using the theory of planned behavior. J Appl Soc Psychol. 1999;29:72–90. [Google Scholar]
- 21.Boudreau F. Godin G. Understanding physical activity intentions among French Candadians with type 2 diabetes: an extension of Ajzen's theory of planned behaviour. In J Behav Nutr Phys Act. 2009;6:35. doi: 10.1186/1479-5868-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishbein M. The role of theory in HIV prevention. AIDS Care. 2000;12:273–278. doi: 10.1080/09540120050042918. [DOI] [PubMed] [Google Scholar]
- 23.Godin G. Valois P. Lepage L. Desharnais R. Predictors of smoking behaviour: an application of Ajzen's theory of planned behaviour. Br J Addict. 1992;87:1335–1343. doi: 10.1111/j.1360-0443.1992.tb02742.x. [DOI] [PubMed] [Google Scholar]
- 24.Robinson N. Masser B. White K. Hyde M. Terry D. Predicting intentions to donate blood among nondonors in Australia: an extended theory of planned behavior. Transfusion. 2008;48:2559–2567. doi: 10.1111/j.1537-2995.2008.01904.x. [DOI] [PubMed] [Google Scholar]
- 25.Van Ryn M. Lutle L. Kirscht J. A test of the theory of planned behavior for two health-related practices. J Appl Soc Psychol. 1996;26:871–883. [Google Scholar]
- 26.Montano D. Taplin S. A test of an expanded theory of reasoned action to predict mammography participation. Soc Sci Med. 1991;32:733–741. doi: 10.1016/0277-9536(91)90153-4. [DOI] [PubMed] [Google Scholar]
- 27.Brubaker R. Fowler C. Encouraging college males to perform testicular self-examination: evaluation of a persuasive message based on the revised theory of reasoned action. J Appl Soc Psychol. 1990;17:1411–1422. [Google Scholar]
- 28.Gummeson L. Jonsson I. Conner M. Predicting intentions and behavior of Swedish 10-16-year-olds at breakfast. Food Qual Prefer. 1997;8:297–306. [Google Scholar]
- 29.Hillhouse J. Adler C. Drinnon J. Turrisi R. Application of Ajzen's theory of planned behavior to predict sunbathing, tanning salon use, and sunscreen use intentions and behaviors. J Behav Med. 1997;20:365–378. doi: 10.1023/a:1025517130513. [DOI] [PubMed] [Google Scholar]
- 30.Ajzen I. The theory of planned behavior. Organ Behav Hum Decis Process. 1991;50:179–211. [Google Scholar]
- 31.Schwarzer R. Sneihotta F. Lippke S, et al. On the Assessment and Analysis of Variables in the Health Action Process Approach: Conducting an Investigation. Berlin: Freie Universeitat Berlin; 2003. [Google Scholar]
- 32.Renner B. Kwon S. Yang B. Social-cognitive predictors of dietary behaviors in South Korean men and women. Int J Behav Med. 2008;15:4–13. doi: 10.1007/BF03003068. [DOI] [PubMed] [Google Scholar]
- 33.Fishbein M. A reasoned action approach to health promotion. Med Decis Making. 2008;28:834–844. doi: 10.1177/0272989X08326092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Naik A. Kallen M. Walder A. Street R. Improving hypertension control in diabetes mellitus: the effects of collaborative and proactive health communication. Circulation. 2008;117:1361–1368. doi: 10.1161/CIRCULATIONAHA.107.724005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalichman S. Cain D. Fuhrel A. Eaton L. Di Fonzo K. Ertl T. Assessing medication adherence self-efficacy among low-literacy patients: development of a pictographic visual analogue scale. Health Educ Res. 2005;20:24–35. doi: 10.1093/her/cyg106. [DOI] [PubMed] [Google Scholar]
- 36.Murphy D. Marelich W. Rappaport N. Hoffman D. Farthing C. Results of an antiretroviral adherence intervention: STAR (staying healthy: taking antiretrovirals regularly) J Int Assoc Physicians AIDS Care. 2007;6:113–124. doi: 10.1177/1545109707301243. [DOI] [PubMed] [Google Scholar]
- 37.Simoni J. Amico K. Smith L. Nelson K. Antiretroviral adherence interventions: translating research findings to the real world clinic. Curr HIV/AIDS Rep. 2010;7:44–51. doi: 10.1007/s11904-009-0037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fumaz C. Munoz–Moreno J. Molto J. Sustained antiretroviral treatment adherence in survivors of the pre-HAART era: attitudes and beliefs. AIDS Care. 2008;20:796–805. doi: 10.1080/09540120701694022. [DOI] [PubMed] [Google Scholar]
- 39.Grimes R. Grimes D. Readiness: the state of the science (or the lack thereof) Curr HIV/AIDS Rep. 2010;7:245–252. doi: 10.1007/s11904-010-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Vellis R. Scale Development: Theory and Applications. Thousand Oaks: Sage Publications; 2003. [Google Scholar]
- 41.Thompson B. Exploratory Factor Analysis Decision Sequence. Washington, DC: American Psychological Assocation; 2004. [Google Scholar]
- 42.Wainberg M. Friedland G. Public health implications of antiretroviral therapy and HIV drug resistance. JAMA. 1998;279:1977–1983. doi: 10.1001/jama.279.24.1977. [DOI] [PubMed] [Google Scholar]
- 43.Samal L. Saha S. Chander G, et al. Internet health information seeking behavior and antiretroviral adherence in persons living with HIV/AIDS. AIDS Patient Care STDS. 2011;24:445–449. doi: 10.1089/apc.2011.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.French T. Tesoriero J. Agins B. Changes in stress, substance use and medication beliefs are associated with changes in adherence to HIV antiretroviral therapy. AIDS Behav. 2011;15:1416–1428. doi: 10.1007/s10461-010-9762-4. [DOI] [PubMed] [Google Scholar]
- 45.Barclay T. Hinkin C. Castellon S, et al. Age-associated predictors of medication adherence in HIV-positive adults: health beliefs, self-efficacy, and neurocognitive status. Health Psychol. 2007;26:40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.