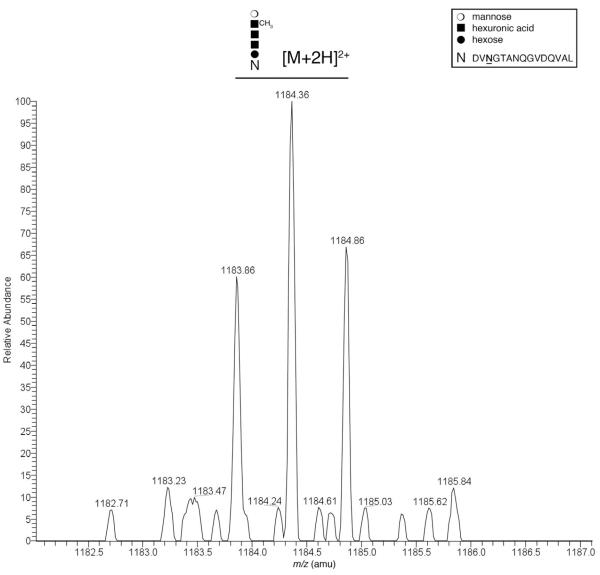
Fig. 4.
The Har. marismortui S-layer protein is modified by the same N-linked pentasaccharide as bound to dolichol phosphate and the Hfx. volcanii S-layer protein. A monoisotopic [M+2H]2+ ion peak corresponding to a Har. marismortui S-layer glycoprotein Asn-467 (amino acid numbering before signal peptide cleavage)-containing chymotrypsin-generated fragment, as detected by LC-ESI/MS. The mass-to-charge ratio of the doubly charged peak observed (1183.86 Da) is in good agreement with calculated mass of the peptide modified by a pentasaccharide comprising two hexoses, two hexuronic acids and the methyl ester of hexuronic acid (1183.8608 Da), schematically depicted above the peak. The inset depicts the symbols used for each sugar residue as well as the peptide sequence represented by ‘N’. The modified Asn residue in the peptide is indicated in bold and underlined.