Abstract
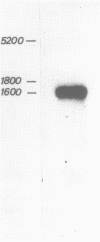
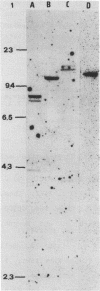
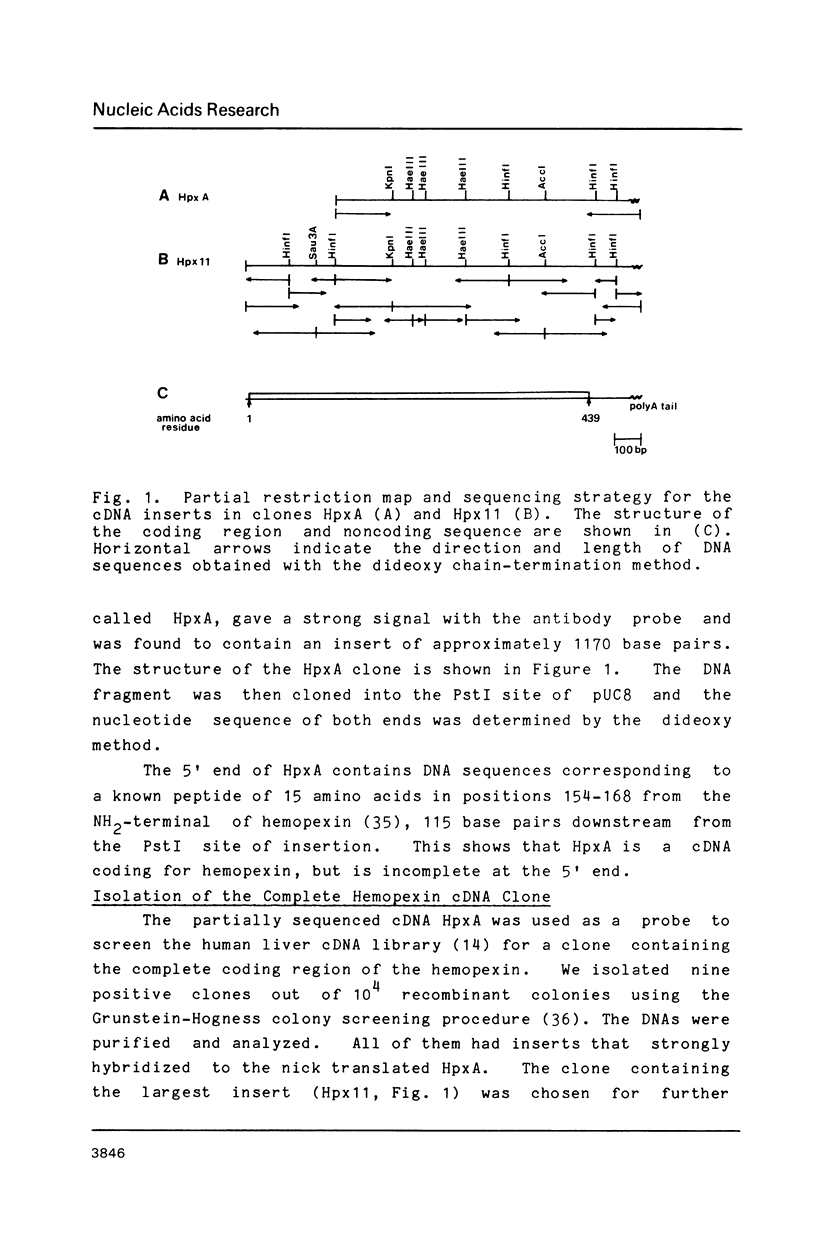
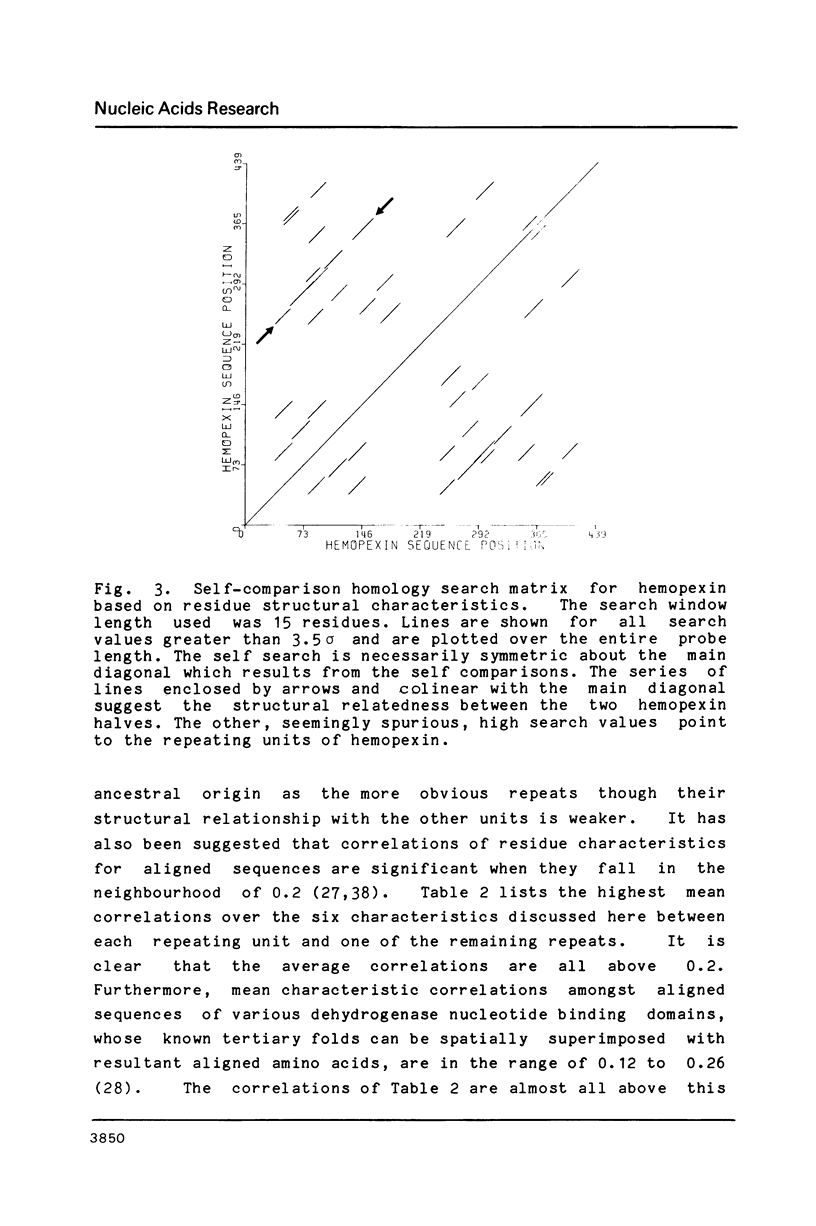
We have cloned and analyzed a cDNA containing the coding sequence for human hemopexin. We have first identified, by immunological screening of 30.000 colonies of a liver cDNA library in the expression vector pEX1, a clone carrying an insert 1170 base pairs long that shows 100% homology with a known human hemopexin peptide. The complete sequence coding for hemopexin was isolated from a liver cDNA library in the vector pAT218. The DNA insert of 1523 base pairs shows an open reading frame coding for 439 amino acids, a 3' noncoding region of 159 nucleotides long, followed by a poly(A) tail. The insert spans the entire coding region and from which the primary structure of the protein was deduced. By computer assisted analysis of the amino acid sequence, it was possible to recognize a core unit, of about 45 amino acids, which is repeated 8 or possibly even 10 fold along the polypeptide chain. This feature suggests that the gene might have evolved through a series of duplications. This characteristic, together with prediction of secondary structure, suggest a rough model for the tridimensional folding that allows some speculations on the function of hemopexin. Blot hybridization of total RNA from human liver with nick translated hemopexin cDNA detected a message of about 1600 nucleotides. Southern blot experiments to identify the hemopexin gene (s) suggest that it is not a large multi-gene family, but that there is only one or at most a few genes in the human genome.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P., Hanei M., Wilson J. M., Kelley W. N. A possible nucleotide-binding domain in the tertiary fold of phosphoribosyltransferases. J Biol Chem. 1983 May 25;258(10):6450–6457. [PubMed] [Google Scholar]
- Argos P., Palau J. Amino acid distribution in protein secondary structures. Int J Pept Protein Res. 1982 Apr;19(4):380–393. doi: 10.1111/j.1399-3011.1982.tb02619.x. [DOI] [PubMed] [Google Scholar]
- Argos P., Schwarz J., Schwarz J. An assessment of protein secondary structure prediction methods based on amino acid sequence. Biochim Biophys Acta. 1976 Aug 9;439(2):261–273. doi: 10.1016/0005-2795(76)90062-3. [DOI] [PubMed] [Google Scholar]
- Baumann H., Jahreis G. P., Gaines K. C. Synthesis and regulation of acute phase plasma proteins in primary cultures of mouse hepatocytes. J Cell Biol. 1983 Sep;97(3):866–876. doi: 10.1083/jcb.97.3.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Walter P., Chang C. N., Goldman B. M., Erickson A. H., Lingappa V. R. Translocation of proteins across membranes: the signal hypothesis and beyond. Symp Soc Exp Biol. 1979;33:9–36. [PubMed] [Google Scholar]
- Broome S., Gilbert W. Immunological screening method to detect specific translation products. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2746–2749. doi: 10.1073/pnas.75.6.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Creighton T. E. Experimental studies of protein folding and unfolding. Prog Biophys Mol Biol. 1978;33(3):231–297. doi: 10.1016/0079-6107(79)90030-0. [DOI] [PubMed] [Google Scholar]
- Danieli G. A., Angelini C. Letter: Duchenne carrier detection. Lancet. 1976 Jul 10;2(7976):90–90. doi: 10.1016/s0140-6736(76)92301-1. [DOI] [PubMed] [Google Scholar]
- Davies D. M., Smith A., Muller-Eberhard U., Morgan W. T. Hepatic subcellular metabolism of heme from heme-hemopexin: incorporation of iron into ferritin. Biochem Biophys Res Commun. 1979 Dec 28;91(4):1504–1511. doi: 10.1016/0006-291x(79)91235-x. [DOI] [PubMed] [Google Scholar]
- Goldberg D. A. Isolation and partial characterization of the Drosophila alcohol dehydrogenase gene. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5794–5798. doi: 10.1073/pnas.77.10.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Diamond D., Wozney J. M., Boedtker H. RNA molecular weight determinations by gel electrophoresis under denaturing conditions, a critical reexamination. Biochemistry. 1977 Oct 18;16(21):4743–4751. doi: 10.1021/bi00640a033. [DOI] [PubMed] [Google Scholar]
- McLachlan A. D. Tests for comparing related amino-acid sequences. Cytochrome c and cytochrome c 551 . J Mol Biol. 1971 Oct 28;61(2):409–424. doi: 10.1016/0022-2836(71)90390-1. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Palau J., Argos P., Puigdomenech P. Protein secondary structure. Studies on the limits of prediction accuracy. Int J Pept Protein Res. 1982 Apr;19(4):394–401. [PubMed] [Google Scholar]
- Pongor S., Szalay A. A. Prediction of homology and divergence in the secondary structure of polypeptides. Proc Natl Acad Sci U S A. 1985 Jan;82(2):366–370. doi: 10.1073/pnas.82.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanzey B., Mercereau O., Ternynck T., Kourilsky P. Methods for identification of recombinants of phage lambda. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3394–3397. doi: 10.1073/pnas.73.10.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seery V. L., Muller-Eberhard U. Binding of porphyrins to rabbit hemopexin and albumin. J Biol Chem. 1973 Jun 10;248(11):3796–3800. [PubMed] [Google Scholar]
- Smith A., Morgan W. T. Hemopexin-mediated transport of heme into isolated rat hepatocytes. J Biol Chem. 1981 Nov 10;256(21):10902–10909. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K., Luzio J. P. Construction of a new family of high efficiency bacterial expression vectors: identification of cDNA clones coding for human liver proteins. EMBO J. 1984 Jun;3(6):1429–1434. doi: 10.1002/j.1460-2075.1984.tb01988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley K. K. Solubilization and immune-detection of beta-galactosidase hybrid proteins carrying foreign antigenic determinants. Nucleic Acids Res. 1983 Jun 25;11(12):4077–4092. doi: 10.1093/nar/11.12.4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffen D., Weinberg R. A. The integrated genome of murine leukemia virus. Cell. 1978 Nov;15(3):1003–1010. doi: 10.1016/0092-8674(78)90284-2. [DOI] [PubMed] [Google Scholar]
- Takahashi N., Nakai S., Honjo T. Cloning of human immunoglobulin mu gene and comparison with mouse mu gene. Nucleic Acids Res. 1980 Dec 20;8(24):5983–5991. doi: 10.1093/nar/8.24.5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Complete amino acid sequence of human hemopexin, the heme-binding protein of serum. Proc Natl Acad Sci U S A. 1985 Jan;82(1):73–77. doi: 10.1073/pnas.82.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi N., Takahashi Y., Putnam F. W. Structure of human hemopexin: O-glycosyl and N-glycosyl sites and unusual clustering of tryptophan residues. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2021–2025. doi: 10.1073/pnas.81.7.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlenbruck G., Reese I., Vaith P., Haupt H. Immuno-chemical studies on the alkali-labile carbohydrate chains of human serum glycoproteins. J Clin Chem Clin Biochem. 1979 Jan;17(1):29–34. doi: 10.1515/cclm.1979.17.1.29. [DOI] [PubMed] [Google Scholar]
- Villa-Komaroff L., Efstratiadis A., Broome S., Lomedico P., Tizard R., Naber S. P., Chick W. L., Gilbert W. A bacterial clone synthesizing proinsulin. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3727–3731. doi: 10.1073/pnas.75.8.3727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods D. E., Markham A. F., Ricker A. T., Goldberger G., Colten H. R. Isolation of cDNA clones for the human complement protein factor B, a class III major histocompatibility complex gene product. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5661–5665. doi: 10.1073/pnas.79.18.5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]