Abstract
We are interested in investigating the biological activity of chalcones, a major class of compounds found in the beverage kava, in order to develop potent and selective chemopreventive candidates. Consumption of kava in the South Pacific Islands is inversely correlated with cancer incidence, even among smokers. Accordingly, chalcones have anti-cancer activities in animal and cell culture models. To investigate signaling pathways that affect chalcone action we studied a potent analog, (E)-3-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (chalcone-24). Chalcone-24 was selected from a series of chalcone analogs that were synthesized based on the structures derived from flavokawain compounds found in kava, and screened in A549 lung cancer cells for induction of cytotoxicity and inhibition of NF-κB, a transcription factor associated with cell survival. Incubation of A549 cells with chalcone-24 resulted in a dose-dependent inhibition of cell viability, inhibition of NF-κB, activation of caspases, and activation of extracellular signal regulated kinase 1/2 (ERK1/2) and c-Jun N-terminal kinase (JNK); ERK1/2 and JNK are mitogen activated protein kinases that play central roles in regulating cell fate. Pharmacological inhibitors of ERK1/2 or JNK increased the sensitivity of A549 cells to chalcone-24-induced cytotoxicity, without affecting NF-κB or caspase activity. These results will help refine the synthesis of chalcone analogs to maximize the combination of actions required to prevent and treat cancer.
Keywords: chalcone, A549, mitogen activated protein kinase, extracellular signal regulated kinase, c-Jun N-terminal kinase, kava
1. Introduction
There is considerable interest in chalcones, a class of naturally occurring flavonoid compounds, as potential therapeutic and chemopreventive agents because they have anti-cancer activity and because they are easy to synthesize [1, 2]. In particular, chalcones are a major class of compounds found in a beverage made from the roots of the kava plant (Piper methysticum); intriguingly, epidemiological studies suggest that populations in the South Pacific Islands that consume the kava beverage, including smokers, have relatively low incidences of cancer [3]. Kava also reduced lung tumor formation in A/J mice treated with the tobacco smoke carcinogens 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and benzo[a]pyrene [4]. In addition, chalcones are cytotoxic to a variety of cancer cell lines [1, 2]. We are interested in investigating the biological activity of chalcones in order to develop potent and selective chemopreventive candidates. Accordingly, a series of chalcone analogs was synthesized whose structures were derived from flavokawain compounds found in kava [5]. The chalcone analogs were screened for the abilities to block the growth of A549 lung cancer cells and inhibit the activity of NF-κB, a transcription factor that is involved in inflammation and cell survival [6]. We chose to study a potent analog from this series, (E)-3-(3-hydroxy-4-methoxyphenyl)-1-(3,4,5-trimethoxyphenyl)prop-2-en-1-one (chalcone-24), to investigate the signaling pathways that modulate the cytotoxicity of chalcone analogs in A549 cells.
We focused on the effects of chalcone-24 on three major members of the mitogen activated protein kinase (MAPK) family that play central roles in regulating cell proliferation and cell death, extracellular signal regulated kinase 1 and 2 (ERK1/2), c-Jun N-terminal kinase (JNK), and p38 [7]. Mitogenic agents such as growth factors typically stimulate ERK1/2 activity. Agents that induce stress, such as proinflammatory cytokines, typically stimulate JNK and p38 activities. Our studies indicate that ERK1/2 and JNK activity help determine the threshold for chalcone-24-induced cytotoxicity in A549 lung cancer cells.
2. Material and methods
2.1 Materials
Chalcone-24 was synthesized as described in [5]. U0126 was purchased from Calbiochem (La Jolla, CA). SP600125 was purchased from AG Scientific (San Diego, CA) or Sigma-Aldrich (St. Louis, MO). RPMI and fetal bovine serum (FBS) were purchased from Invitrogen Corporation (Carlsbad, CA). Other materials were purchased from Sigma-Aldrich (St. Louis, MO).
2.2. Cell Culture
A549 cells were purchased from the American Type Culture Collection (Manassas, VA) and grown in RPMI media supplemented with 10% FBS in a humidified incubator at 37°C with 5% CO2. Cells were plated at 5000 cells per well in 96 well plates for cell proliferation assays, 2 × 106 cells per 100 mm plate for nuclear extracts, and at 3 × 105 cells per 35 mm plate for all other assays. The day after plating, the cells were incubated in the absence or presence of the compounds indicated in the figure legends.
2.3. Cell proliferation
Cell number was determined with the use of a CellTiter 96® Non-Radioactive Cell Proliferation Assay (MTT) (Promega), according to the manufacturer's instructions and the methods described in [8]. All readings were on the linear portion of the standard curve.
2.4. TNF-α-induced NF-κB activation
The assay for NF-κB activation was conducted essentially as described in [5]. Briefly, A549 cells stably transfected with NF-κB-luc were purchased from Panomics (Freemont, Ca). One day prior to each experiment A549 NF-KB-luc cells were plated at a density of 2 × 104 cells/well in 24-well plates. NF-κB activation was stimulated by incubating the cells with 20 ng/mL recombinant human TNF-α (R&D Systems, Minneapolis, MN). Cells were then washed, lysed and luciferase activity assayed according to the protocol for the Luciferase Assay System recommended by Promega (Madison, WI). Relative light units were measured using a Monolight 3010 Luminometer (Pharmingen). Luciferase data was normalized to total protein levels determined using a Bradford Assay (Bio-Rad, Hercules, CA).
2.5. Caspase-3/7 activation
Caspase-3/7 activation was determined by using the Caspase-Glo 3/7 Assay according to the protocol recommended by Promega (Madison, WI). Briefly, cells were plated at a density of 3 × 103 cells/well in 96-well plates one day prior to each experiment. Cells were incubated in complete media without phenol red in the absence or presence of the compounds indicated in the figure legends. After the appropriate exposure time an equal volume of Caspase-Glo 3/7 reagent was added to each well. The plate was then incubated for 30 minutes at room temperature. Luminescence was read using a BioTek Synergy HT plate reader (Winooski, VT)
2.6. Antibodies and immunoblotting
Cell lysates and nuclear extracts were prepared and immunoblotting conducted as described in [9]. After blocking in a TBST/5% milk solution, immunoblots were incubated overnight at 4°C using the following primary antibodies and dilutions: phospho-p44/42 MAPK (Thr-202/Tyr-204) (E10) (mouse monoclonal) (1:2000), phospho-SAPK/JNK (Thr-183/Tyr 185) (G9) (mouse monoclonal) (1:5000), anti-SAPK/JNK (56G8) (rabbit monoclonal) (1:2000), and phospho-c-Jun (Ser-73) (D47G9) XP rabbit monoclonal (1:2000) (3270) from Cell Signaling (Beverly, MA), and ERK2 (C-14) (rabbit polyclonal) (1:500), and c-Jun (N) (1:1000), from Santa Cruz Biotechnology (Santa Cruz, CA). The secondary antibodies, anti-mouse IgG horseradish peroxidase-linked antibody and anti-rabbit IgG horseradish peroxidase-linked antibody, were purchased from Cell Signaling. Immunoblots were visualized using the Pierce SuperSignal West Pico or Fempto substrate.
2.7. Statistical analyses
Statistical analyses were performed using GraphPad Prism version 4.0 for Macintosh. Statistical significance was assessed as indicated in the figure legends using a standard 1-way analysis of variance (ANOVA) or 2-way ANOVA, and the Bonferroni post-test.
3. Results and discussion
3.1. Chalcone-24 inhibits A549 cell viability and activation of NF-κB
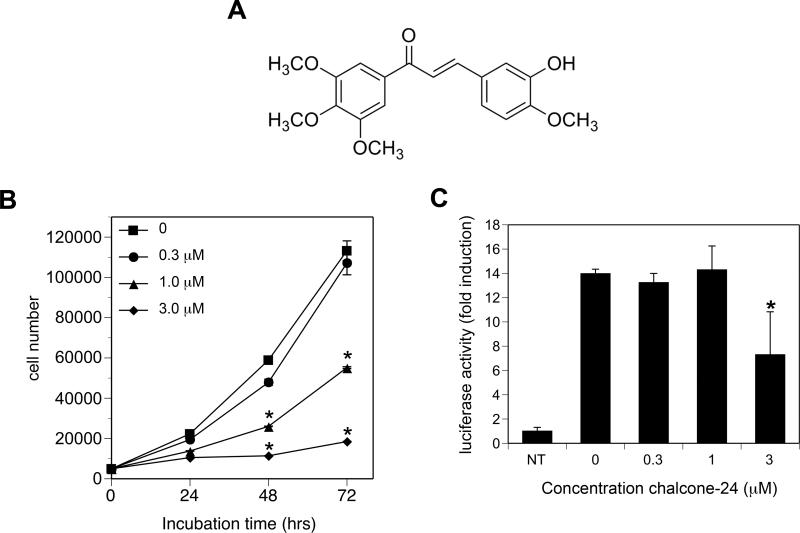
To investigate signaling pathways affected by chalcone-24 (Fig. 1A), we first determined the dose-response for chalcone-24-induced inhibition of A549 cell viability (Fig. 1B) and NF-κB activation (Fig. 1C). Incubation of A549 cells with 1 μM and 3 μM chalcone-24 induced a significant decrease in cell viability by 48 and 72 hours (Fig. 1B). By 72 hours, the viability of cells incubated with 1 μM and 3 μM chalcone-24 was approximately 50% and 20% of control cells, respectively (Fig. 1B). Cell viability was similar in control cells and A549 cells incubated with 0.3 μM chalcone-24 over 72 hours (Fig. 1B, compare circles and squares). TNF-α-stimulated NF-κB activation was significantly inhibited by 3 μM chalcone-24, but not 0.3 μM or 1 μM chalcone-24 (Fig. 1C). These results indicate that 1 μM chalcone-24 can inhibit A549 cell viability without prior inhibition of NF-κB-dependent cell survival pathways.
Fig. 1. Chalcone-24 inhibits A549 cell proliferation.
(A) Structure of chalcone-24. (B) A549 cells were incubated for 24, 48, or 72 hours without (squares) or with the following concentrations of chalcone-24: 0.3 μM (circles), 1 μM (triangles), or 3 μM (diamonds). Cell number was determined by an MTT assay as described in Materials and methods. The zero time point represents the number of cells that were initially plated. The symbols represent the average of triplicates ± SEM. Asterisks denote values that were determined to be statistically significantly different from cells incubated without chalcone-24 (p < 0.001) by using a 1-way ANOVA and Bonferroni post-test. The results of the MTT assays were confirmed by counting live and dead cells using trypan blue exclusion (data not shown). (C) A549 cells stably transfected with NF-κB-luc were incubated for 8 hours without (NT) or with TNF-α in the presence of the indicated concentrations of chalcone-24. NF-κB activity was determined as described in Materials and methods. The bars represent the average of triplicates ± SEM. The values represent fold induction of luciferase activity relative to non-treated controls (NT). Asterisks denote values that were determined to be statistically significantly different from cells incubated with TNF-α but without chalcone-24 (p < 0.001) by using a 1-way ANOVA and Bonferroni post-test. The data shown are representative of at least two independent experiments.
3.2. Chalcone-24 stimulates the activation of ERK1/2 and JNK
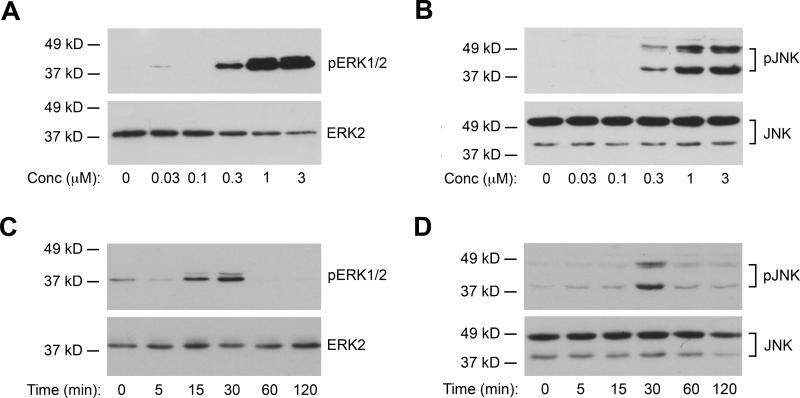
Next, we investigated the effects of chalcone-24 on ERK1/2, JNK, and p38 activity (Fig. 2). ERK1/2 and JNK activity were monitored by immunoblot analysis of the phosphorylated, active forms of the kinases (Figs. 2A and 2B). Concentrations as low as 0.3 μM chalcone-24 stimulated detectable increases in the levels of the phospho-ERK1/2 and phospho-JNK (Figs. 2A and 2B). Incubation of A549 cells with 0.3 μM chalcone-24 stimulated transient increases in ERK1/2 and JNK phosphorylation (Figs. 2C and 2D). Increases in phospho-ERK1/2 and phospho-JNK phosphorylation were detected by 15 minutes (Fig. 2C) and 30 minutes (Fig. 2D), respectively. Both phospho-ERK1/2 (Fig. 2C) and phospho-JNK levels (Fig. 2D) returned to basal levels by 60 minutes (Fig. 2C). Chalcone-24 did not simulate a detectable increase in the phosphorylated, active form of p38, even when cells were incubated with 3 μM chalcone throughout an 8-hour period (data not shown).
Fig. 2. Chalcone-24 stimulates ERK1/2 and JNK activity in A549 cells.
(A) and (B) A549 cells were incubated for 30 minutes with the indicated concentrations of chalcone-24. Whole cell lysates were analyzed by immunoblot for (A) phosphorylated, active ERK1/2 (top panel, 20 μg protein) and total ERK2 (bottom panel, 10 μg protein) or (B) phosphorylated, active JNK1 and JNK2 (top panel, 40 μg protein) and total JNK (bottom panel, 10 μg protein). (C) and (D) A549 cells were incubated for the indicated times with 0.3 μM chalcone-24. Whole cell lysates were analyzed by immunoblot for (C) phosphorylated, active ERK1/2 (top panel 30 μg protein) and total ERK1/2 (bottom panel, 10 μg protein) or (D) phosphorylated, active JNK1 and JNK2 (top panel, 40 μg protein) and total JNK (bottom panel, 10 μg protein). The data shown are representative of at least two independent experiments.
3.3. Inhibition of ERK1/2 or JNK increases the sensitivity of A549 cells for chalcone-24-induced cytotoxicity
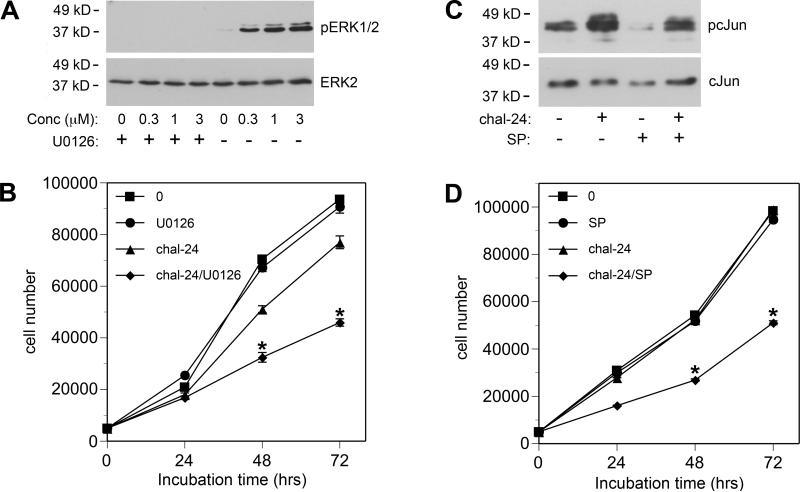
Because 0.3 μM chalcone-24 stimulated activation of ERK1/2 and JNK, but induced a minimal loss of cell viability, we wanted to determine if activation of these kinases protect A549 cells from chalcone-24-induced cytotoxicity. We used pharmacological inhibitors of ERK1/2 and JNK to investigate how these kinases affect the action of 0.3 μM chalcone-24. We blocked chalcone-24-stimulated ERK1/2 activation by incubating the cells with U0126, which inhibits MEK1/2, the kinases that phosphorylate and activate ERK1/2 [10]. Incubation of A549 cells with 3 μM U0126 inhibited chalcone-24-induced ERK1/2 activation (Fig. 3A). The cell viability of A549 cells incubated for 72 hours in the presence of both 0.3 μM chalcone-24 and 3 μM U0126 was over 40% lower than the cell viability of A549 cells incubated with 0.3 μM chalcone-24 alone (Fig. 3B, compare diamonds and triangles). These results indicate that inhibition of ERK1/2 increases the sensitivity of A549 cells to chalcone-24-induced decrease in cell viability (Fig. 4B).
Figure 3. Inhibition of ERK1/2 or JNK increases the sensitivity of A549 cells to chalcone-24.
(A) A549 cells were incubated for 30 minutes in the absence (-) or presence (+) of 3 μM U0126. The cells were then incubated for 30 minutes in the absence (-) or presence (+) of the indicated concentrations of chalcone-24. ERK1/2 activity was analyzed as described in the legend to Figure 2. (B) Twenty-four hours after plating A549 cells, the cells were incubated in absence (squares) or presence of 3 μM U0126 (circles), 0.3 μM chalcone-24 (chal-24) (triangles), or 0.3 μM chalcone-24 and 3 μM U0126 together (chal-24/U0126)(diamonds). Cell number was determined by MTT assay after 24, 48, and 72 hours of incubation. (C) A549 cells were incubated for 30 minutes in the absence (-) or presence (+) of 5 μM SP600125 (SP). The cells were then incubated for 30 minutes in the absence (-) or presence (+) of 0.3 μM chalcone-24 (chal-24). Protein from nuclear extracts (5 μg) was analyzed for either phospho-c-Jun by immunoblot using an antibody that binds to c-Jun phosphorylated on Ser-73 (pc-Jun) (upper panel) or total c-Jun (lower panel). (D) Twenty-four hours after plating A549 cells, the cells were incubated in absence (squares) or presence of 5 μM SP600125 (SP) (circles), 0.3 μM chalcone-24 (chal-24) (triangles), or with 0.3 μM chalcone and 5 μM SP600125 (chal-24/SP) together (diamonds). Cell number was determined by MTT assay after 24, 48, and 72 hours of incubation. (B) and (D) The symbols represent the average of triplicates ± SEM. Asterisks denote values from cells treated with chalcone in the presence of U0126 or SP600125 that were determined to be statistically significantly different from cells incubated with chalcone-24 alone (p < 0.001), by using a 2-way ANOVA and the Bonferroni post-test. The data shown are representative of at least two independent experiments.
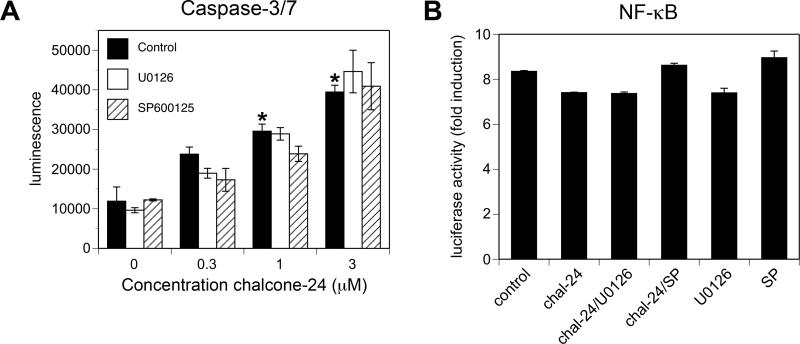
Fig. 4. Inhibition of ERK1/2 or JNK does not lower the threshold for chalcone-24-induced cell death or inhibition of TNF-α-stimulated NF-κB activation.
(A) A549 cells were incubated for 48 hours in the presence of the indicated concentrations of chalcone-24, in the absence (closed bars) or presence of 3 μM U0126 (open bars) or 5 μM SP600125 (hatched bars). Caspase-3/7 activity was measured as described in Materials and methods. The bars represent the average of triplicates ± S.D. Asterisks above the closed bars denote values that were determined to be statistically significantly different from cells incubated in the absence of chalcone-24 (p < 0.001) by using a 1-way ANOVA and Bonferroni post-test. Caspase-3/7 activity was not significantly different between cells treated in the presence of U0126 or SP600125 and cells treated in the absence of these pharmacological inhibitors. (B) A549 cells stably transfected with NF-κB-luc were incubated for 8 hours with TNF-α in the absence (control) or presence of 0.3 μM chalcone-24 (chal-24), 0.3 μM chalcone-24 plus 3 μM U0126 (chal-24/U0126), 0.3 μM chalcone-24 plus 5 μM SP600125 (chal-24/SP), 3 μM U0126 (U0126), or 5 μM SP600125 (SP). NF-κB activity was determined as described in Materials and methods. The values represent fold induction of luciferase activity relative to cells that were not incubated with TNF-α. The bars represent the average of triplicates ± S.D. The values were determined to be not statistically significantly different from cells incubated with TNF-α alone by using a 1-way ANOVA and Bonferroni post-test. U0126 and SP600125 alone or together with 0.3 μM chalcone-24 did not inhibit TNF-α-stimulated NF-κB-luc when the cells were incubated for 24 hours (data not shown). The data shown are representative of at least two independent experiments.
Likewise, we found that inhibition of JNK increases the sensitivity of A549 cells to chalcone-24-induced inhibition of A549 cell viability. We assessed endogenous JNK activity by monitoring the phosphorylation state of endogenous c-Jun, a substrate for JNK, with immunoblot analysis (Fig. 3C, upper panel). Incubation of A549 cells with 5 μM SP600125 blocked basal and chalcone-24-induced JNK activation by approximately 40%, as assessed by densitometry of immunoblot data. Concentrations of SP600125 higher than 5 μM were toxic to A549 cells (data not shown). Preincubating the cells with 5 μM SP600125 did not block chalcone-24-stimulated ERK1/2 activation (data not shown). The cell viability of A549 cells incubated for 72 hours in the presence of both 0.3 μM chalcone-24 and 5 μM SP600125 was over 48% lower than the cell viability of A459 cells incubated with 0.3 μM chalcone alone (Fig. 3D, compare diamonds and triangles). Inhibiting ERK1/2 and JNK activation resulted in a modest increase in the ability of 1 μM chalcone-24 to reduce A549 cell viability and had no effect on the action of 3 μM chalcone-24 (data not shown). Altogether, these data indicate that inhibition of ERK1/2 and JNK lowers the threshold for chalcone-24-induced inhibition of cell viability.
3.4. Inhibition of ERK1/2 or JNK does not lower the threshold for chalcone-24-induced cell death or inhibition of TNF-α-stimulated NF-κB activation
To determine whether the decrease in cell viability observed in chalcone-24-treated A549 cells was due, a least in part, to the stimulation of cell death pathways, we used an assay that can detect caspase-3 and caspase-7 activity, which are markers of apoptosis [11]. Incubation of A549 cells for 48 hours with 1 μM or 3 μM chalcone-24 stimulated a significant increase in caspase activity, indicating that stimulation of apoptotic pathways requires concentrations of chalcone-24 greater than 0.3 μM (Fig. 4A). We detected an increase in caspase activity as early as 24 hours of incubation, but were not able to detect an increase at 16 hours (data not shown). Staining the cells with propidium iodide followed by flow cytometry confirmed that the threshold for stimulation of cell death is between 0.3 μM and 1 μM chalcone-24 (data not shown). Preincubation of A549 cells with either U0126 or SP600125 did not affect caspase activity, indicating that ERK1/2 and JNK do not modulate chalcone-24-induced caspase-3- and capsase-7-dependent cell death in A549 cells (Fig. 4A). The use of trypan blue exclusion to monitor cell viability confirmed that inhibition of ERK1/2 or JNK does not increase cell death (data not shown). Incubating A549 cells with 0.3 μM chalcone-24 did not inhibit TNF-α-stimulated NF-κB activity, regardless of the presence or absence of 0.3 μM chalcone-24, U0126, and SP600125 (Fig. 4B). Altogether, these data indicate that inhibition of ERK1/2 or JNK lowers the threshold for the chalcone-24-induced decrease in cell viability without lowering the threshold for chalcone-24-induced-activation of cell death pathways or inhibition of NF-κB-dependent cell survival pathways.
3.5. Conclusions
The studies presented here indicate that ERK1/2 and JNK activity help determine the sensitivity of A549 cells to the cytotoxic effects of chalcone-24. Our results suggest that chalone-24 induces cytotoxicity through three different mechanisms, depending on the dose: inhibition of cell proliferation, stimulation of apoptotic cell death pathways, and inhibition of NF-κB-dependent cell survival pathways. Accordingly, kava both blocked cell proliferation and induced apoptosis in the A/J mouse model of lung tumorigenesis [4]. Interestingly, ERK1/2 and JNK appear to primarily protect cells from the chalcone-24-induced pathways that inhibit cell proliferation, as inhibition of these kinases did not affect caspase or NF-κB activity. This may help explain why ERK1/2 and JNK activity protect A549 cells from 0.3 μM chalcone-24, which does not stimulate cell death or inhibit NF-κB. By contrast, ERK1/2 and JNK activity does not protect against the cytotoxic effects of 3 μM chalone-24 (data not shown), a concentration that can act through all three cytotoxic pathways. Interestingly ERK1/2 and JNK activity only modestly protects A549 cells from the cytotoxic effects of 1 μM chalcone-24 (data now shown), a concentration that inhibits cell proliferation and activates apoptotic cell death pathways, and does not inhibit NF-κB. These results suggests that the protective effects of ERK1/2 and JNK activity can be overcome when more than one cytotoxic pathway is activated by chalcone-24.
Further research is required to determine the biochemical mechanisms by which chalcone-24 stimulates ERK1/2 and JNK activity, and the biochemical targets of ERK1/2 and JNK that modulate cytotoxicity. Studies indicate that specific chalcones stimulate ERK1/2 through a pathway that involves elevation of cAMP, activation of cAMP-dependent protein kinase (PKA) and activation of MEK1/2 [12]. We found that the PKA inhibitor H-89 did not block chalcone-24-stimulated ERK1/2 activation of A549 cells, suggesting that chalcone-24 does not require PKA to activate ERK1/2 in this cell line (data not shown). A common theme for the action of many chalcones is effects on the cell cycle (reviewed in [2]). Further research is needed to determine whether chalcone-24 targets cell cycle regulators through the activation of ERK1/2 and JNK.
Altogether, our results suggest that the sensitivity of A549 lung cancer cells to growth inhibition by chalcone derivatives is determined by the constellation of signaling pathways and cytotoxicity pathways modulated by chalcone analogs. This information will help guide future studies to further investigate the mechanisms of action of chalcone derivatives and to refine the synthesis of chalcone analogs to maximize the combination of actions required to prevent and treat cancer.
Highlights for Inhibition of mitogen activated protein kinases increases the sensitivity of A549 lung cancer cells to the cytotoxity induced by a kava chalcone analog.
A kava chalcone analog induces cytotoxicity in A549 lung cancer cells
Inhibitors of ERK1/2 and JNK increase A549 cell sensitivity to the chalcone analog
ERK1/2 and JNK modulate antiproliferative effects, but not cell death or survival
Acknowledgements
We thank Ameeta Kelekar and Xazmin Lowman for their assistance with the various assays for cell death. This research was supported by National Institutes of Health grant RO1-CA104609 (E.V.W), a grant from the University of Minnesota AHC Faculty Research Development Program: 2010 (to E.V.W.), National Institutes of Health grant R03-CA125844 (C.X.), and an Engebretson Drug Design & Development Grant 2010 (C.X). These funding sources were not involved in study design; the collection, analysis and interpretation of data; the writing of this report; or the decision to submit this report for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
There are no conflicts of interest.
References
- 1.Go ML, Wu X, Liu XL. Chalcones: an update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005;12:481–499. doi: 10.2174/0929867053363153. [DOI] [PubMed] [Google Scholar]
- 2.Boumendjel A, Ronot X, Boutonnat J. Chalcones derivatives acting as cell cycle blockers: potential anti cancer drugs? Curr. Drug Targets. 2009;10:363–371. doi: 10.2174/138945009787846416. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal R, Deep G. Kava, a tonic for relieving the irrational development of natural preventive agents. Cancer Prev. Res. 2008;1:409–412. doi: 10.1158/1940-6207.CAPR-08-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnson TE, Kassie F, O'Sullivan MG, Negia M, Hanson TE, Upadhyaya P, Ruvolo PP, Hecht SS, Xing C. Chemopreventive effect of kava on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo[a]pyrene-induced lung tumorigenesis in A/J mice. Cancer Prev. Res. 2008;1:430–438. doi: 10.1158/1940-6207.CAPR-08-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srinivasan B, Johnson TE, Lad R, Xing C. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3',4',5'-tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J. Med. Chem. 2009;52:7228–7235. doi: 10.1021/jm901278z. [DOI] [PubMed] [Google Scholar]
- 6.Perkins ND. The diverse and complex roles of NF-kappaB subunits in cancer. Nat. Rev. Cancer. 2012;12:121–132. doi: 10.1038/nrc3204. [DOI] [PubMed] [Google Scholar]
- 7.Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene. 2007;26:3279–3290. doi: 10.1038/sj.onc.1210421. [DOI] [PubMed] [Google Scholar]
- 8.Wichmann AE, Thomson NM, Peterson LA, Wattenberg EV. Genotoxic methylating agents modulate extracellular signal regulated kinase activity through MEK-dependent, glutathione-, and DNA methylation-independent mechanisms in lung epithelial cells. Chem. Res. Toxicol. 2003;16:87–94. doi: 10.1021/tx0256026. [DOI] [PubMed] [Google Scholar]
- 9.Zeliadt NA, Warmka JK, Wattenberg EV. Mitogen activated protein kinases selectively regulate palytoxin-stimulated gene expression in mouse keratinocytes. Toxicol. Appl. Pharmacol. 2003;192:212–221. doi: 10.1016/s0041-008x(03)00298-9. [DOI] [PubMed] [Google Scholar]
- 10.English JM, Cobb MH. Pharmacological inhibitors of MAPK pathways. Trends Pharmacol. Sci. 2002;23:40–45. doi: 10.1016/s0165-6147(00)01865-4. [DOI] [PubMed] [Google Scholar]
- 11.Kuribayashi K, Mayes PA, El-Deiry WS. What are caspases 3 and 7 doing upstream of the mitochondria? Cancer Biol. Ther. 2006;5:763–765. doi: 10.4161/cbt.5.7.3228. [DOI] [PubMed] [Google Scholar]
- 12.Al Rahim M, Nakajima A, Misawa N, Shindo K, Adachi K, Shizuri Y, Ohizumi Y, Yamakuni T. A novel diol-derivative of chalcone produced by bioconversion, 3-(2,3-dihydroxyphenyl)-1-phenylpropan-1-one, activates PKA/MEK/ERK signaling and antagonizes Abeta-inhibition of the cascade in cultured rat CNS neurons. Eur. J. Pharmacol. 2008;600:10–7. doi: 10.1016/j.ejphar.2008.09.046. [DOI] [PubMed] [Google Scholar]