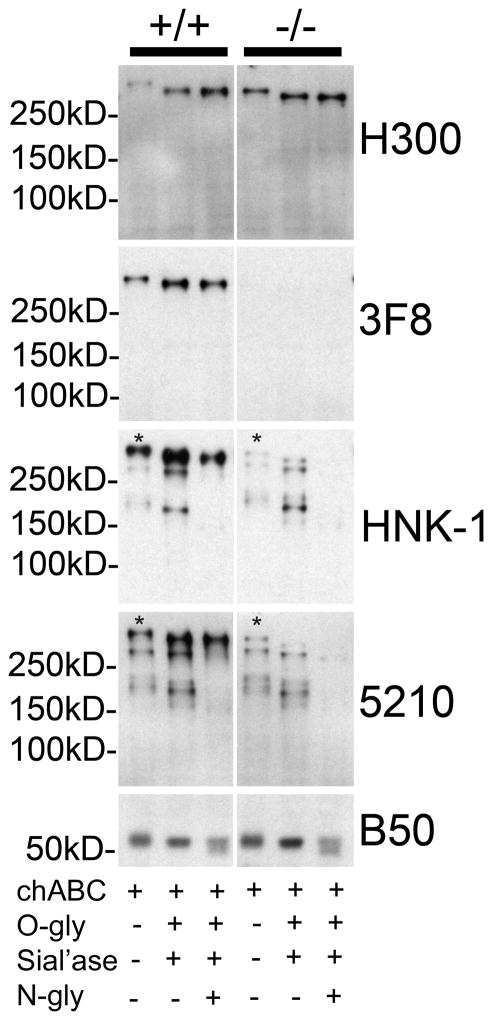
FIGURE 4. Residual HNK-1 glycan reactivity on RPTPζ/phosphacan in the cortex of P4 POMGnT1 knockout animals is N-linked.
Soluble fractions from P4 POMGnT1 +/+ and −/− cortical homogenates were subject to enzymatic protein deglycosylation with chABC and combinations of O-glycosidase (O-gly), sialidase (Sial’ase), and N-glycanase (N-gly) and then subject to Western blot analysis. Electrophoresis on 7% SDS- polyacrylamide gels and immunoblotting of +/+ samples with H300 revealed a reactive band that when treated with chABC alone ran well above 250kD and was the same molecular weight as the 3F8 reactive band in the corresponding sample. Enzymatic deglycosylation with chABC, O-gly, sial’ase resulted in a shift toward a lower molecular weight and an increase in the intensity of H300 reactivity in both +/+ and −/− samples. The same was also observed for 3F8 in +/+ samples, while no 3F8 reactivity was detected in POMGnT1 −/− samples. High molecular weight bands reactive for HNK-1 and 5210 which resolved at the same molecular weight as H300 and 3F8 reactive bands in +/+ samples treated with chABC (denoted with an *), corresponding to phosphacan/RPTPζ, were decreased in intensity in POMGnT1 −/− samples. Enzymatic deglycosylation with a combination of chABC, O-gly, sial’ase, and N-gly resulted in a near complete elimination of these bands (*) in POMGnT1 −/− samples in comparison to chABC, O-gly, sial’ase alone. Additionally, numerous bands of lower molecular weight also reactive for HNK-1 and 5210 were detected in chABC treated wildtype samples and found to be unaltered in POMGnT1 −/− samples. The reactivity of these lower molecular weight bands, which likely detect proteins other than RPTPζ/phosphacan, were essentially eliminated with the treatment of chABC, O-gly, sial’ase, and N-gly relative to chABC, O-gly, sial’ase alone. Subsequently, a single band corresponding to RPTPζ/phosphacan in the +/+ samples remained and essentially all reactive bands in POMGnT1 −/− samples were nearly eliminated, as faint residual bands were detected at longer exposures in these samples (not shown). The B50 antibody against the cleavage fragment of brevican served as the internal control for both total protein concentration and enzymatic deglycosyaltion.