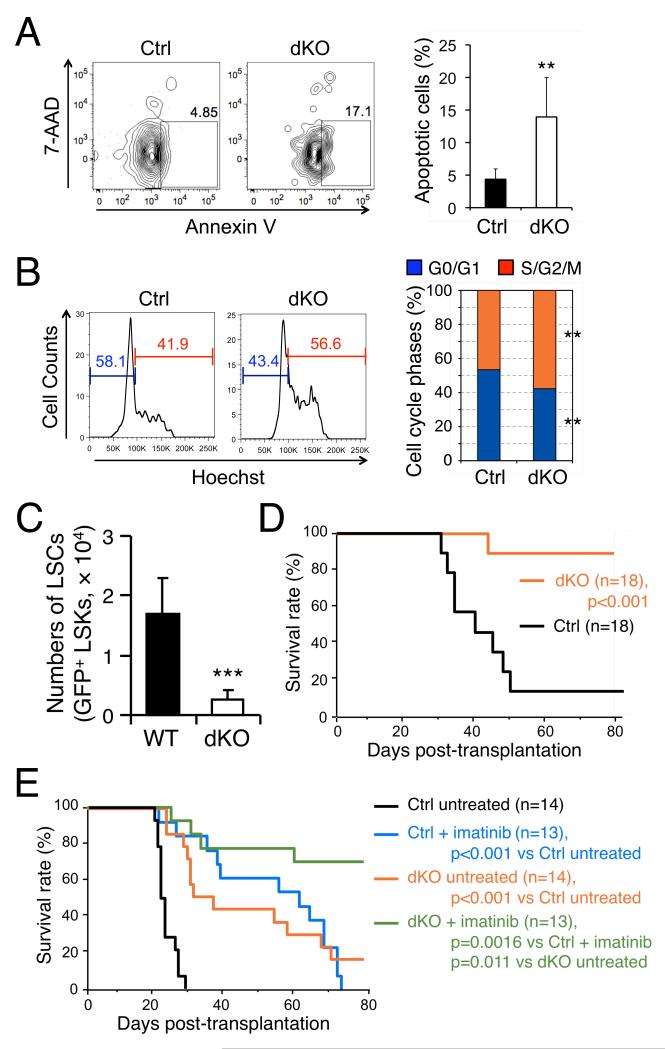
Figure 6. Targeting GABPβ1L/β2 impairs LSC self-renewal and synergizes with imatinib therapy to eradicate CML.
(A) Intrinsic requirement of GABPβ1L/β2 for LSC survival. Fifteen to twenty days after transplantation of p210BCR-ABL-infected cells, BM cells from the primary recipients were surface-stained to identify LSCs (GFP+ LSKs) and further stained for AnnexinV and 7-AAD. The percentage of AnnexinV+7-AAD− apoptotic cells is shown in representative contour plots and summarized in bar graph on the right (n = 4 for control, and n = 8 for dKO). **, p<0.01. (B) LSCs lacking GABPβ1L/β2 exhibit more active cycling. BM cells isolated from the primary recipients as in (A). To avoid quenching of GFP in LSCs, the cells were first incubated with Hoechst33342 at 37oC for 45 min, followed by two washes with HBSS and surface staining on ice. Percentages of cells in G0/G1 or S/G2/M phases are shown in representative histograms and summarized in bar graph on the right (n = 4 for Ctrl, and n = 8 for dKO). **, p<0.01. (C) Enumeration of LSCs in primary recipients. Primary recipients of p210BCR-ABL-transduced BM cells were sacrificed on day 15 post-BMT, and GFP+ LSK cells in the BM were determined. Representative data from 3 independent experiments are shown. ***, p<0.001. (D) CML propagation in secondary recipients. GFP+ LSK cells were sorted from primary recipients of p210BCR-ABL-transduced BM cells on day 15 post-BMT. Ten thousand sorted cells were injected into another cohort of syngeneic mice along with 2 × 105 protectors, and CML progression was monitored. Data in (D) and (E) are pooled results from 2 independent experiments, and statistical significance was assessed using log-rank test. (E) Synergistic effect of GABPβ1L/β2 deficiency and imatinib therapy in controlling CML in vivo. Primary recipients of p210BCR-ABL-transduced BM cells were untreated or treated with imatinib (100 mg/kg body weight, b.i.d.) from day 8 to day 80 post-BMT, with CML progression monitored.