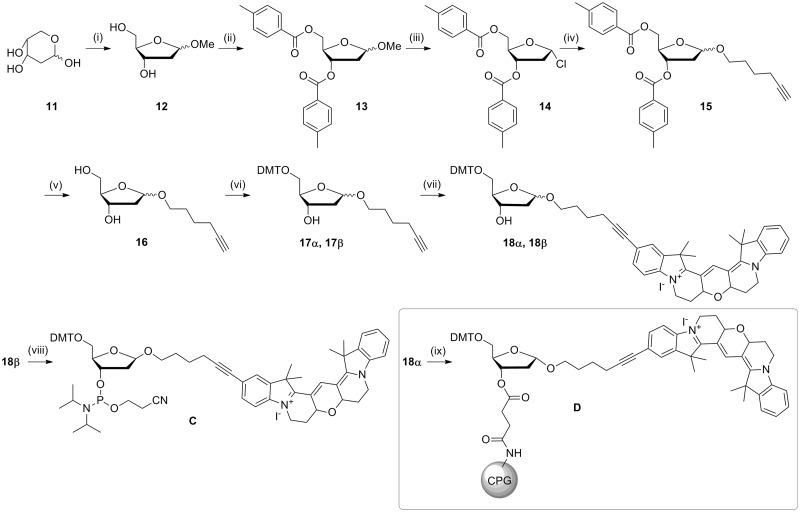
Scheme 4.
Synthesis of β-Cy3BdR phosphoramidite and α-Cy3BdR solid support. Reagents and conditions; (i) MeOH, 1% methanolic HCl, room temperature, 30 min; (ii) pyridine, p-toluoyl-chloride, 0°C then room temperature, 16 h; (iii) acetic acid, saturated HCl in acetic acid, acetyl chloride, 0°C then room temperature, 64% over three steps; (iv) THF, DCM, DMAP, 5-hexyn-1-ol, room temperature, 3.5 h, 53%; (v) methanolic ammonia, 45°C, 16 h, 82%; (vi) DMTCl, pyridine, DMAP, 1 h, room temperature, α 47%, β 41% (total 88%); (vii) 7, CuI, Pd(PPh3)4, Et3N, DMF, 52 h, room temperature, α 66%, β 73%; (viii) 2-cyanoethyl-N,N-diisopropyl-chlorophosphoramidite, DIPEA, DCM, room temperature, 45 min, 94%; (ix) succinylated amino-link resin, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride, DMAP, Et3N.