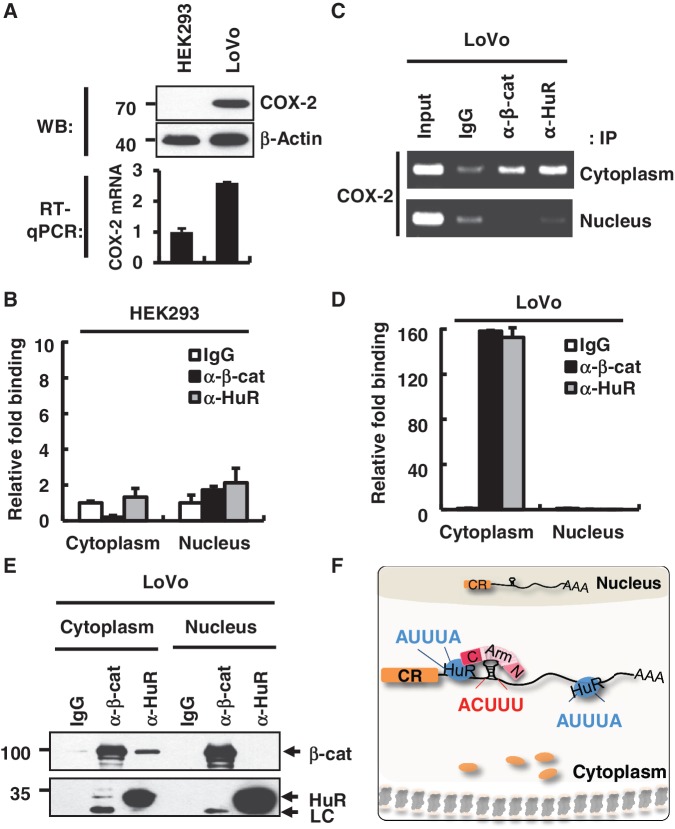
Figure 5.
Differential binding patterns of HuR and β-catenin on the COX-2 3′-UTR in normal and tumor cells. (A) Upper panel, western blot analysis of the COX-2 protein in HEK293 and LoVo cells. β-Actin was used as a loading control. Molecular weights are indicated on the left side of the gel. Lower panel, RT–qPCR analysis was performed to analyze the endogenous COX-2 mRNA level in HEK293 and LoVo cells. (B) RNP-IP assay with fractionated HEK293 cells. RNP-IP was performed with anti-β-catenin or anti-HuR antibodies. RT–qPCR analysis was performed to analyze the bound pellet RNA and presented as relative fold binding to the IgG control. Three independent samples were analyzed, and the average and standard deviation are shown. (C) RNP-IP assay with LoVo cells as in B. RT–PCR analysis was performed for the bound pellet RNA and the input RNA. (D) Real-time PCR analysis of RNP-IP pellet RNA with LoVo cells as in C. (E) Co-IP of β-catenin and HuR from nuclear and cytoplasmic fractions of LoVo cells. Protein size markers are shown on the left side of the gel. (F) A model for the β-catenin–HuR–COX-2 mRNA tertiary RNP formation in cancer cells. β-Catenin and HuR bind to the 3′-UTR of COX-2 mRNA via distinct RNA elements, ACUUU and AUUUA, respectively. β-Catenin and HuR may interact with each other through the C-term domain of the β-catenin protein. The tertiary RNP complex was exclusively found in the cytoplasm of colorectal adenocarcinoma cells and promoted COX-2 protein expression in cancer cells. CR, coding region of COX-2 mRNA; black line: 3′-UTR of COX-2 mRNA; blue circle, HuR protein; N, N-terminal domain of β-catenin; Arm, Armadillo domain of β-catenin; C, C-terminal domain of β-catenin; AAA, poly A tail; orange circle, Cyclooxygenase-2 protein.