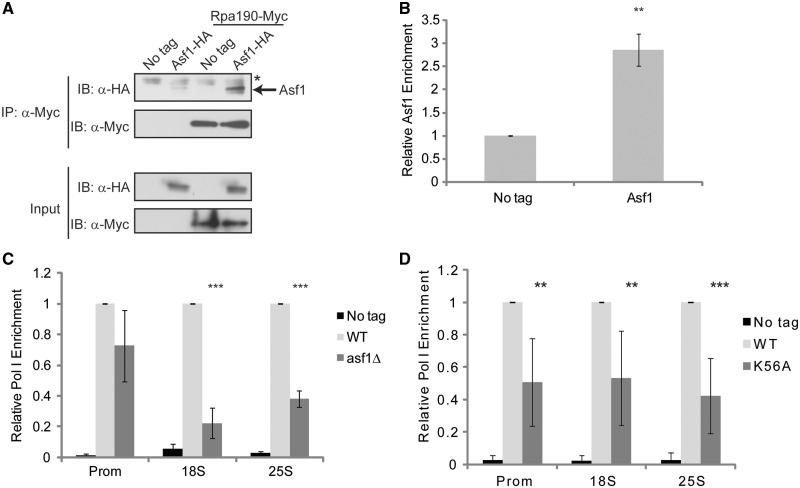
Figure 6.
The H3K56ac pathway regulates rDNA transcription. (A) Asf1 coassociates with RNA Pol I. WCE from the indicated log phase cultures were prepared and α−Myc immunoprecipitations were performed using 750 µg of WCE. Samples were resolved by 10% SDS–PAGE, immunoblotted with α−HA antibody and then membranes were stripped and reprobed with α−Myc antibody. Input samples represent 30 µg of each extract. The asterisk denotes a cross-reactive protein found in all the samples. (B) Asf1 localizes to the rDNA promoter. ChIP experiments using α-HA antibody and either no tag control or Asf1-6XHA expressing cells were performed using the rDNA promoter primer set indicated in Figure 3A. The Asf1-specific signal was expressed as fold increase over the no tag signal which was set to 1. The average and standard deviation of three independent experiments are presented and significance was determined by t-test. **P < 0.05. (C) Asf1 regulates RNA Pol levels on the rDNA. α-Myc ChIP was performed using no tag control, Rpa190-Myc or Rpa190-Myc asf1Δ strains and the level of RNA Pol I determined at the indicated positions on the rDNA. The wild-type Rpa190-Myc signal was set to 1 and the no tag control and asf1Δ signals were expressed relative to this signal. The average and standard deviation of three or more independent experiments are shown with significance determined by t-test. ***P < 0.01. (D) Loss of H3K56ac reduces RNA Pol I recruitment to the rDNA. No tag control, histone H3 wild-type or H3K56A strains expressing Rpa190-9XMyc were used in α−Myc ChIP as described in (C). The histone H3 wild-type strain was set to 1 for each primer set and the no tag control and H3K56A mutant expressed relative to it. The average and standard deviation of four to five independent experiments are shown with significance determined by t-test. **P < 0.05; ***P < 0.01.