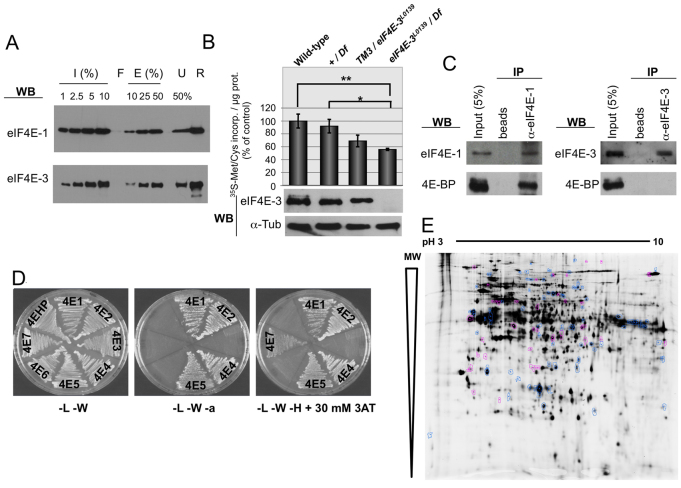
Fig. 5.
eIF4E-3 promotes translation in testis. (A) m7GTP-Sepharose pull-down. Recombinant eIF4E-1 and eIF4E-3 proteins were subjected to m7GTP-Sepharose chromatography. Input (I), flow-through (F), eluted (E) and unbound (U) fractions, as well as the resin (R) after elution, were subjected to SDS-PAGE and western blotting to detect the indicated proteins. (B) Translation in testis as measured by [35S]Met/Cys incorporation ex vivo. (Top) [35S]Met/Cys incorporation of testes of the indicated genotypes. The results of three independent experiments are shown with s.d. Extremely significant (**, P<0.0005) and significant (*, P=0.0186) differences (Student’s t-test, GraphPad) were found between eIF4E-3 loss-of-function [eIF4E-3L0139/Df(3L)BSC732] and wild-type and +/Df(3L)BSC732 values, respectively. (Bottom) The presence of eIF4E-3 and α-tubulin (loading control) in the indicated testes was analyzed by western blotting, probing 20 μg/lane total protein extracts. (C) Co-immunoprecipitation (co-IP) experiments showing that 4E-BP physically interacts with eIF4E-1 but not with eIF4E-3 in testis. Total testis extracts were used to conduct immunoprecipitations using either beads alone or beads plus the indicated antibodies in the presence of RNase A, and interactions were detected by immunoblotting. (D) 4E-BP interacts with eIF4E-1, -2, -4 and -5, but not with eIF4E-3, in the yeast two-hybrid system. 4E-HP and eIF4E-6 were used as negative controls. L, leucine; W, tryptophan; a, adenine; H, histidine; 3AT, 3-amino-1,2,4-triazole. (E) Comparison of testis protein extracts from wild-type and eIF4E-3 mutant flies by 2D-DiGE. Out of ∼2000 protein spots, 120 differ by at least 1.5-fold between wild type and mutant (t-test, P≤0.01). A mixture of wild-type and mutant proteins was run as an internal standard on one of the gels; spots that exhibit an increase in the mutant (38/120) are indicated in magenta, spots that are decreased (82/120) are shown in blue.