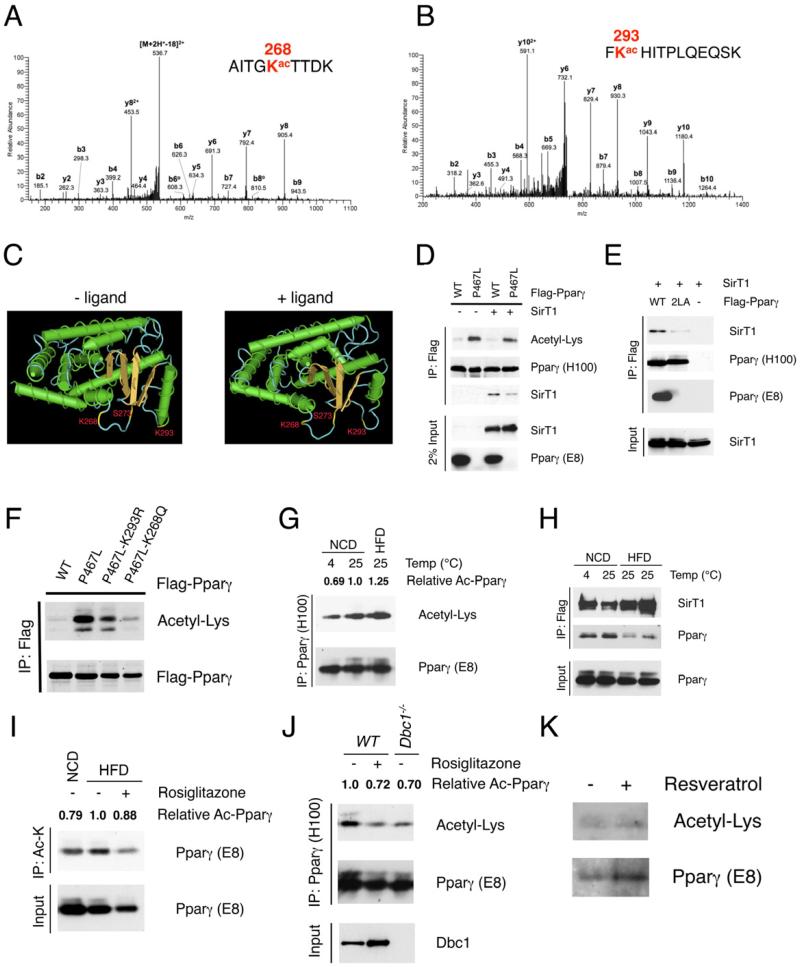
Figure 5. Identification of Pparγ Lys268 and Lys293 as SirT1 substrates.
(A-B) Annotation of MS/MS spectra of acetylated peptides of Pparγ after trypsin digestion at Lys268 (A) and at Lys293 (B).
(C) 3-D model of liganded and unliganded Pparγ structure generated by NIH Cn3D software, localizing Lys268, Lys293 and Ser273 within helix 2-helix 2’ region.
(D) Decreased SirT1 binding and increased acetylation of Pparγ P467L mutant. Co-IP of Flag-tagged WT or P467L mutant Pparγ with SirT1 in 293 cells. Pparγ (E8) antibody fails to recognize P467L mutant.
(E) Mutation of the LxxLL motif on helix 12 disrupts SirT1 binding. Co-IP of Flag-tagged WT or 2LA mutant Pparγ with SirT1 in 293 cells.
(F) Mutations of Lys268 or Lys293 decrease acetylation of P467L mutant Pparγ in 293 cells.
(G) Pparγ acetylation in pooled iWAT from 4-5 male mice exposed to 4 °C overnight or fed HFD for 16 weeks. We used 12 mg protein to immunoprecipitate Pparγ using antibody H100.
(H) Interaction of Pparγ with SirT1 in iWAT. Pooled iWAT from 3-5 male SirBACO mice exposed to 4 °C overnight or fed HFD for 16 weeks. We used 8 mg protein as in each lane to co-IP Flag-tagged SirT1.
(I) Pparγ acetylation in response to TZD following HFD. Male WT mice were fed HFD for 18 weeks and treated with rosiglitazone for 3 days. Pooled iWAT from 4 mice was lysed, and 12 mg protein was used in each lane to immunoprecipitate with acetyl-Lysine (Ac-K) antibody.
(J) Pparγ acetylation in response to TZD and deletion of Dbc1. Chow-fed male WT mice were treated with rosiglitazone for 3 days. 12 mg protein from pooled iWAT of 4 mice was used for IP with Pparγ antibody (H100).
(K) Pparγ acetylation in human adipose tissue in response to resveratrol. Human subcutaneous adipose fragments were treated with resveratrol (50 μM) for 12 hours. 1.2 mg protein was immunoprecipitated with Pparγ antibody (H100).
We estimated relative levels of Pparγ acetylation (Ac-Pparγ) using densitometry with NIH ImageJ software.
See also Figure S5.