Abstract
Phosphatidylethanolamine is an important inner-leaflet phospholipid, and CTP:phosphoethanolamine cytidylyltransferase-Pcyt2 acts as the main regulator of the de novo phosphatidylethanolamine synthesis from ethanolamine and diacylglycerol. Complete deletion of the mouse Pcyt2 gene is embryonic lethal, and the single-allele deficiency leads to development of the metabolic syndrome phenotype, including liver steatosis, hypertriglyceridemia, obesity, and insulin resistance. This study aimed to specifically elucidate the mechanisms of hypertriglyceridemia in Pcyt2 heterozygous mice (Pcyt2+/−). Evidence here shows that unlike 8 week-old mice, 32 week- and 42 week-old Pcyt2+/− mice experience increased VLDL secretion and liver microsomal triglyceride transfer protein activity. Older Pcyt2+/− mice also demonstrate increased levels of postprandial plasma TAGs, increased stimulation of genes responsible for intestinal lipid absorption, transport and chylomicron secretion, and dramatically elevated plasma Angptl4, apoB-100, and apoB-48 content. In addition, plasma HL and LPL activities and TAG clearance following a lipid challenge were significantly reduced in Pcyt2+/− mice relative to control littermates. Collectively, these results establish that the hypertriglyceridemia that accompanies Pcyt2 deficiency is the result of multiple metabolic adaptations, including elevated hepatic and intestinal lipoprotein secretion and stimulated expression and/or activity of genes involved in lipid absorption and transport and lipoprotein assembly, together with reduced plasma TAG clearance and utilization with peripheral tissues.
Keywords: phospholipid, very low density lipoprotein secretion, chylomicron formation, triglyceride
Lipid biosynthesis is essential for the maintenance of cell function and energy homeostasis and defects in lipid metabolism contribute to chronic diseases, including metabolic syndrome, atherosclerosis, and type 2 diabetes. Many studies have also demonstrated that perturbed glucose and FA metabolism are significant risk factors in the development of these pathologies. In contrast, our understanding of how membrane phospholipids contribute in the development of chronic disease is considerably less studied and it is generally poorly understood. There have been select lines of evidence that have highlighted the relationship between phospholipids, mainly phosphatidylcholine (PC) and triglyceride (TAG) metabolism (1–5). FAs released from phospholipid degradation can be utilized for TAG synthesis (2) and changes in membrane PC content are sufficient to cause changes in whole body TAG homeostasis (2, 3). Furthermore, mutations lowering CTP:phosphocholine cytidylyltransferase (Pcyt1) activity in the PC-Kennedy pathway in Chinese hamster ovary cells result in a redirection of diacylglycerol (DAG) from phospholipids to TAG (3). In Drosophila, inhibition of PC synthesis increases TAG content in lipid droplets by altering the size and the morphology of the droplets (1). Evidence from the mouse model with deleted phosphatidylethanolamine (PE) methylation (PEMT) pathway shows that reduced PC synthesis and choline availability could prevent development of high-fat diet-induced obesity (as reviewed in Refs. 4 and 5), and that reduced PC-to-PE membrane ratio contributed to the development of liver steatosis (6, 7) and the endoplasmic reticulum stress in obesity (7).
The specific interaction between PE and TAG metabolism has been largely unexplored. Our laboratory has recently described a mouse model with genetically reduced PE de novo synthesis (8) which, similar to the inhibition of PC de novo synthesis in Chinese hamster ovary cells (3), leads to elevated DAG and TAG. Mammalian PE could be synthesized in mitochondria by decarboxylation of phosphatidylserine (PS), but the majority of PE is produced de novo from ethanolamine and DAG through the PE–Kennedy pathway (8–10). In this pathway, ethanolamine is first phosphorylated by ethanolamine kinase to phosphoethanolamine. CTP-phosphoethanolamine cytidylyltransferase (Pcyt2) then catalyzes the formation of CDP-ethanolamine from phosphoethanolamine and CTP, and CDP-ethanolamine and DAG form PE in the final step of the pathway.
Pcyt2 is highly specific and the main regulatory enzyme in the PE–Kennedy pathway (9). Pcyt2 is encoded by a single gene and exists in two catalytically active isoforms, created by alternative splicing (11, 12). Human and murine Pcyt2 are regulated at the transcriptional level by CAAT-box proteins, oxysterols, and LXR, and stimulatory proteins Sp1 and Sp3. Human Pcyt2 is additionally regulated by LXR, EGR1, and NFκB and is downregulated in breast cancer (13–15). Complete deletion of Pcyt2 in mice is embryonic lethal (8), which confirms the essentiality of this gene for animal growth and development. Interestingly, heterozygous mice (Pcyt2−/+) mice (16, 17), as well as Pcyt2 liver-specific knockout mice (18), develop liver steatosis, which establishes a strong physiological connection between PE and TAG syntheses through the common intermediate DAG. To eliminate the excess DAG formed by Pcyt2 gene deletion, both knockout models synthesize additional FAs from glucose by lipogenesis, and animals inevitably accumulate TAG (16, 18). Further consequences of Pcyt2 deletion in the systemic heterozygous (Pcyt2+/−) mice (16) and the liver-conditional knockout (Pcyt2-/-) mice (18) appear to be deficiencies in PUFAs, typically prevalent in PE, and accumulation of saturated FAs and MUFAs in TAG as a consequence of upregulated lipogenesis. The liver conditional Pcyt2-/- mice also have unmodified plasma lipids (even slightly reduced plasma TAG), suggesting impairments in the liver lipoprotein secretion (18). In the heterozygous Pcyt2+/− state, mice have reduced PE synthesis in all tissues, which manifests as a chronic development of metabolic syndrome: the appearance of hepatic steatosis, hypertriglyceridemia, and peripheral insulin resistance at adult stage (16).
The current study was designed to establish the underlying mechanism for the elevated Pcyt2+/− plasma TAG (hypertriglyceridemia) that only develops with heterozygous Pcyt2 deficiency. Contrary to expectations, we demonstrate that Pcyt2+/− mice have modest (32 week) and highly elevated (42 week) hepatic VLDL secretion, which was previously reported as impaired in the liver-specific Pcyt2 knockout mice (18). Here, we describe alternative processes that could contribute the Pcyt2+/− hypertriglyceridemia, such as liver and intestinal TAG absorption/secretion and postprandial TAG turnover. We demonstrate that Pcyt2+/− hypertriglyceridemia is a result of facilitated secretion of both fasting and postabsorptive lipids and an impaired lipolysis and clearance of TAG-rich particles from the circulation. The present investigation contributes new knowledge as to the importance of proper Pcyt2 function in lipid metabolism and whole-body plasma TAG homeostasis, which may help in the development of new strategies for hypertriglyceridemia.
MATERIALS AND METHODS
Animals
The Pcyt2+/− mice of a mixed genetic background (C57Bl/6 × 129/Sv) were generated as described previously (16). Mice were housed under standard conditions with a 12 h light cycle (7.00 AM–7.00 PM), were fed a regular chow diet (Harlan Teklad S-2335), and were given free access to food and water. Experiments were performed on 8, 32, and 42 week-old animals (n = 4–6), after overnight fasting, with food withdrawal at 8.00 PM. The Animal Care Committee of the University of Guelph approved all animal protocols.
Liver VLDL-TAG secretion
Liver TAG secretion was determined in young (8 week) and old (32 week and 42 week) animals. Pcyt2+/− and control littermates were injected intravenously with 500 mg/kg of 10% poloxamer 407 (P407) in sterile saline to block plasma LPL activity (19, 20). Blood was sampled via the saphenous vein at baseline, 1, 2, 3, and 4 h, plasma was isolated, and total TAG was determined using standard protocols (Wako Chemicals and Sigma). The rates of hepatic TAG secretion in Pcyt2+/− and control littermates were compared by linear regression analysis.
Oral lipid load tolerance test
Pcyt2 +/− and wild-type littermates were fasted overnight and were given an intragastric load of 200 µl of olive oil. The mice were anesthetized with isofluorane, and blood was collected at different time points via the retro-orbital plexus immediately after the lipid load and 1–6 h after the load. Plasma TAG content was determined using a standard kit from Sigma. Differences in TAG turnover between two genotypes were determined by integration of TAG content during the entire postload period, after which the differences in the area under the curve (AUC) were compared between the genotypes.
Intestinal chylomicron-TAG secretion
Pcyt2+/− and wild-type littermates were injected intravenously with 500 mg/kg of 10% P407. Subsequently, mice were given an intragastric load of 10 µCi of [3H] triolein (TO) in 200 µl of olive oil. Blood samples were collected via the retro-orbital plexus immediately after the lipid load (15 min) and 1, 2, and 3 h after the load. Lipids were extracted from plasma according to the method of Bligh and Dyer (21). [3H]TAG lipids were separated from other components by TLC using a solvent system of heptane-diethyl ether-acetic acid (60:40:3), and the [3H ]radioactivity in the TAG fractions was determined by liquid scintillation counting (LSC) as previously described (16).
Analysis of intestinally secreted lipids
Pcyt2+/− and wild-type littermates were injected with 500 mg/kg of 10% P407 and were given an intragastric [3H]TO fat load as above. Lipids were extracted from the small intestinal mucus at 30 min and 3 h, and from 1 h plasma, according to the method of Bligh and Dyer (21). [3H] radiolabeled TAG, DAG, FFAs, total cholesterol, and total phospholipids were separated and characterized by LSC as we have previously described (16, 17).
Analysis of intestinal lipids
TAG and DAG content of Pcyt2+/− and wild-type intestinal mucosa was determined after isolation and separation on Silica gel-60 TLC plates using hexane-diethyl ether-acetic acid (60:40:3 v/v) and visualization with iodine vapor. TAG and DAG content in Pcyt2-deficient mice relative to wild-type mice were determined by densitometry (16). Total intestinal TAG content (nmol/mg) was determined by the TAG fluorometric assay kit from Abcam.
Plasma clearance of TAG-rich particles
Radiolabeled TAG-rich particles were prepared by sonication of 75 µCi of [3H]TO in 100 mg of a lipid emulsion containing 23:70:2:3:2 v/v ratio of PC, “cold” triolein, lyso-PC, cholesteryl oleate, and cholesterol (22, 23). The sonicated lipid particles were stored at 4°C and used within 7 days after preparation. Degradation of the radiolabeled TAG-rich particles was followed in Pcyt2+/− and wild-type littermates after an intravenous injection of 100 µg of the radiolabeled emulsion. The blood (50 µl) was collected at 2, 5, 10, 15, and 30 min after injection. Total plasma [3H] radioactivity was determined as described above and expressed as a fraction of the injected dose of the [3H]TO-labeled particles (100%) (22, 23).
Tissue uptake of TAG-rich particles
Lipid distribution into various tissues was determined at the end of the plasma clearance assay, 30 min after the [3H]TO injection described above. Various tissues (liver, heart, muscle, adipose, kidney, spleen) from Pcyt2+/− mice and wild-type littermates were collected, weighed and dissolved in Soluene (PerkinElmer) by an overnight incubation at 70°C (23). The [3H] radioactivity was determined in identical amounts of homogenized tissues. The incorporated [3H] activity was expressed as a % of the injected dose/g weight and compared between the genotypes as described (23).
Plasma LPL and HL activity assays
Fasted Pcyt2+/− mice and wild-type littermates were injected via the retro-orbital plexus with 0.1 U/g of heparin, and the postheparin plasma was collected after 30 min. HL and LPL activities were determined as described (22, 23). The radiolabeled substrate was prepared by sonication of 2.5 µCi/ml [3H]TO with “cold” TO (4.6 mg/ml), FA-free BSA (20 mg/ml), Triton X-100 (0.1%), and heat-inactivated human serum in 0.1 M Tris-HCl buffer (pH 8.6). Ten microliters of the mouse plasma was incubated with 0.2 ml of the sonicated substrate for 30 min at 37°C in the presence (HL activity) and in the absence (total lipase activity) of 1 M NaCl. NaCl inhibits LPL activity and has only a minor effect on HL activity. The reaction was stopped by 3 ml of heptane-methanol-chloroform (1:1.3:1.4) and diluted with 1 ml of 0.1 M K2CO3/saturated boric acid buffer (pH 10.5). The water phase, which contained the released product [3H]oleate, was separated by centrifugation (5 min at 3,000 rpm), and 0.5 ml of the water fraction was counted. The HL activity was calculated as part of total activity that was not inhibited by NaCl, whereas the LPL activity represented the remaining activity. Both activities were expressed as [oleate] nmol/h/ml.
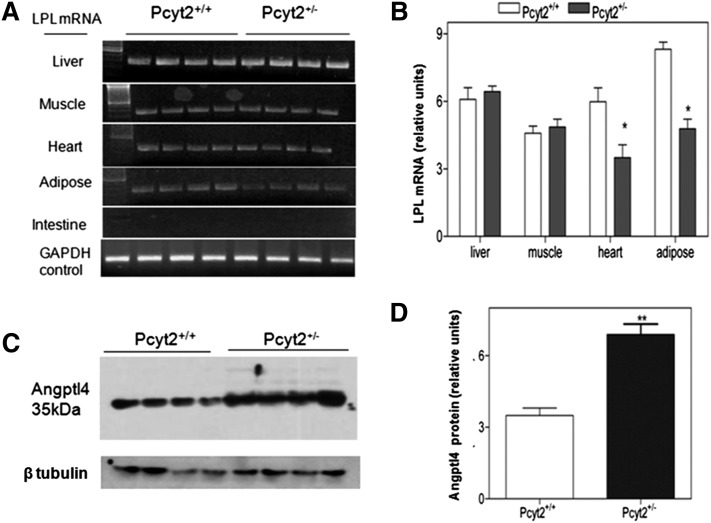
Expression analysis of LPL and angiopoietin-like protein 4
Total mRNA was isolated from 50 mg of homogenized tissues (liver, muscle, heart, intestine, and adipose) using Trizol reagent (Invitrogen). First-strand cDNA was generated from 2 µg of total RNA, and PCR was performed using the LPL-specific primers 5′-GCTCGCACGAGCGCTCCATT-3′ (forward) and 5′-CCTCGGGCAGGGTGAAGGGAA-3′ (reverse). A G3PDH PCR product was used as internal control. Angiopoietin-like protein 4 (Angptl4) was determined in 10 µg of total plasma by Western blotting using an Angptl4-specific antibody (#40-9800) from Invitrogen. The band intensities of the LPL PCR product and Angptl4 protein were analyzed by the ImageJ software.
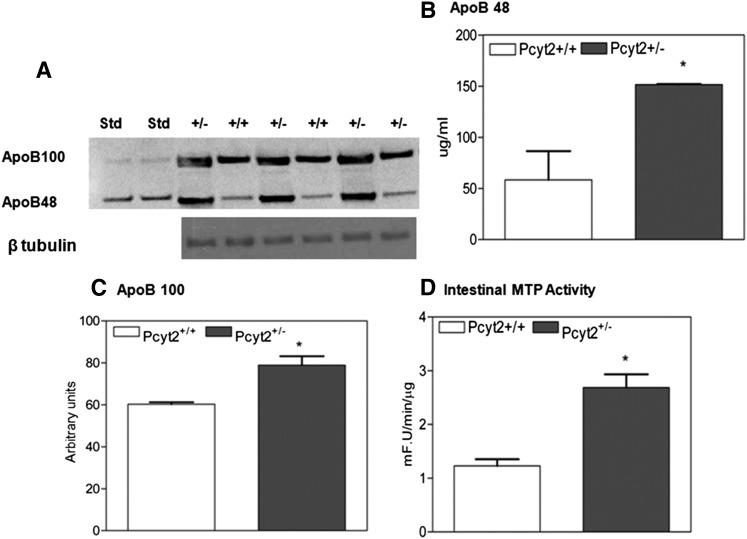
Determination of plasma apoB-48 content
Pcyt2+/− and wild-type littermates were fasted overnight. Subsequently, mice were given an intragastric load of 200 µl of olive oil, and blood samples were drawn 3 h after gavage via the retro-orbital plexus. The plasma was snap-frozen and stored at −80°C until analysis. Plasma apoB-48 concentration was quantified using an adapted Western immune blot method, as previously described (24). Briefly, total plasma was separated by SDS-PAGE on a 3–8% tris-acetate polyacrylamide NuPage® gel (InVitrogen). Separated proteins were transferred to a polyvinylidene fluoride membrane (0.45 μM; ImmobilonP™, Millipore). Membranes were incubated with a goat polyclonal antibody to apo-B (1:100; Santa Cruz Biotech). Detection was achieved using an anti-goat secondary antibody and chemiluminescence (ECL Advance; Amersham Biosciences, UK); intensity was quantified using linear densitometric comparison with a known mass of purified rodent apoB-48 protein.
MTP activity assay
Liver and small intestine from Pcyt2+/− mice and control littermates were homogenized in 10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1 mM EDTA, and 2% protease inhibitor cocktail (Sigma-Aldrich) (25). The microsomal triglyceride transfer protein (MTP) activity was measured using a fluorometric activity assay from Roar Biomedical.
Intestinal gene expression
The small intestine from Pcyt2+/− mice and control littermates was excised and washed with cold isotonic saline solution to remove excess blood. The intestinal lumen was flushed with saline to remove digested food particles, the wall was scraped out with a glass slide, and mucosal tissue was collected in liquid nitrogen. Total RNA isolation and cDNA synthesis was performed as above. Specific primers to measure the expression of intestinal genes MTP, CD46, FATP4, FAS, DGAT1/2, MGAT2, and SREBP1 were used. The PCR conditions and the gene-specific primers used in this analysis can be obtained upon request.
Preparation of radiolabeled enterocytes
Pcyt2+/− mice and wild-type littermates were fasted overnight and given an intragastric load of 10 μCi of [3H]TO in 200 μl of olive oil. The enterocytes were isolated from the intestinal lumen 15 min after the lipid load, as previously described (26–28). The intestinal lumen was first washed with Solution-I (115 mM NaCl, 5 mM KCl, 0.96 mM NaH2PO4, 26 mM NaHCO3, and 5.5 mM glucose, pH 7.4) and gassed with 95% O2 for 20 min. The small intestine was then filled up with Solution-II (67.5 mM NaCl, 1.5 mM KCl, 0.96 mM NaH2PO4, 26.19 mM NaHCO3, 27 mM sodium citrate, and 5.5 mM glucose, pH 7.4) and incubated at 37°C in oxygenated 0.9% saline, with constant shaking. The luminal solution was discarded after 15 min of incubation, and the small intestine was filled up with Solution III (115 mM NaCl, 5 mM KCl, 0.96 mM NaH2PO4, 26 mM NaHCO3, 1.5 mM EDTA, 5.5 mM glucose, 0.5 mM dithiothreitol, pH 7.4), and again incubated for 15 min in aerated 0.9% saline. The luminal content was then collected and centrifuged (5 min; 1,500 rpm; room temperature), and the isolated enterocyte pellet was resuspended in the aerated DMEM. To collect secreted lipoproteins, enterocytes were incubated in the fresh DMEM for 3 h. The lipoprotein particles secreted from enterocytes were collected from the media by sequential density gradient ultracentrifugation, and the radiolabeled lipids in each lipoprotein fraction were determined by LSC.
Density centrifugation of in vitro-secreted intestinal lipoproteins
Sequential density gradient centrifugation was performed on the lipoproteins isolated from enterocytes 15 min after the fat load, and the lipoproteins were secreted from the isolated enterocytes after 3 h incubation. Separation of the large chylomicrons (CML), small chylomicrons (CMSM), and the VLDL-like chylomicron particles (CMVLDL) was performed as previously described (28–30). Media or enterocyte protein homogenates were mixed with 2 ml of 1.006 g/ml-density solution containing 0.57 g/ml KBr, to obtain a final density of 1.10 g/ml. The mixture was overlaid with 3 ml each of 1.063 g/ml- and 1.019 g/ml-density solution and 2 ml of 1.006 g/ml-density solution and subjected to sequential centrifugation. Large chylomicrons (CML) were obtained from the top 1 ml layer after the first centrifugation (33 min, 40,000 rpm, at 15°C using the SW41 rotor). The remaining solution was overlaid with 1 ml of fresh 1.006 g/ml solution and subjected to the second ultracentrifugation (3.5 h, 40,000 rpm, 15°C). The second top 1 ml layer contained the small chylomicron (CMSM) particles. After adding a new 1 ml of 1.006 g/ml solution, the samples were subjected to the third ultracentrifugation (17.5 h, 40,000 rpm, 15°C), of which the 1 ml top fraction represented the CMVLDL (d<1.006 g/ml) particles. The rest of the gradient was fractionated into 1.5 ml portions. CM fractions 1–3 represented the range similar to the LDL size (CMLDL; d = 1.02–1.063 g/ml), and fractions 4–6 represented the range of the HDL (1.063–1.1 g/ml). Total [3H] lipids in all fractions was determined by LSC and the lipoprotein profiles compared between Pcyt2+/− and wild-type littermates.
Density centrifugation of in vivo-secreted intestinal lipoproteins
Plasma from Pcyt2+/− and wild-type littermates was collected 1 h after the [3H]TO lipid load, and 100 µl was subjected to density gradient centrifugation as described above for the in vitro experiments. Total [3H] radioactivity in various lipoprotein fractions was determined by LSC and the distribution profiles compared between Pcyt2+/− and wild-type littermates as described above.
Statistical analysis
Results were expressed as mean ± SD. Statistical analysis was done using the Student paired t-test, the exponential curve fit, and the linear regression analysis using Graph Pad Prism software. Values of P < 0.05 were considered statistically significant.
RESULTS
Liver TAG secretion was elevated in older Pcyt2+/− mice
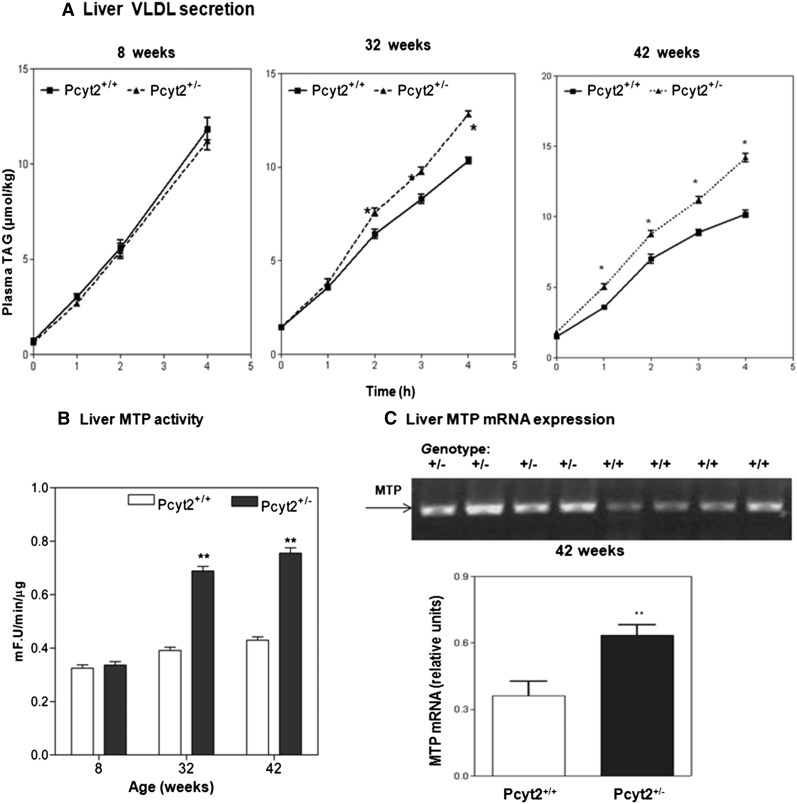
We demonstrated previously that Pcyt2+/− mice develop insulin resistance, elevated plasma VLDL particles, and hypertriglyceridemia at 32–36 weeks of age (16). Young Pcyt2+/− mice (8 week-old) had normal plasma glucose, insulin, and lipoprotein content (16). Therefore, we first investigated whether the VLDL secretion was a contributing factor to the increased plasma TAG observed in older Pcyt2+/− mice. Plasma TAG content (Fig. 1A) and liver MTP activity and expression (Fig. 1B, C) were determined in 8, 32, and 42 week-old Pcyt2+/−mice. VLDL secretion was not significantly different between the 8 week-old Pcyt2+/− mice and wild-type littermates. At 32 weeks of age, however, Pcyt2+/− mice showed an increased TAG secretion, which was further elevated at 42 weeks of age (Fig. 1A). Liver MTP activity and expression correspondingly increased 1.8–2-fold in 32 and 42 week-old Pcyt2+/− mice while remaining normal in 8 week-old Pcyt2+/− mice (Fig. 1B, C). These results collectively established that Pcyt2 deficiency produced an age-dependent upregulation of VLDL secretion that contributed to the elevated plasma VLDL and hypertriglyceridemia in older Pcyt2+/− mice (16). All additional experiments were performed with the hypertriglyceridemic 42 week-old Pcyt2+/−mice.
Fig. 1.
Age-dependent stimulation of VLDL secretion and liver MTP activity in Pcyt2+/−mice. A: Fasting plasma TAG was measured in 8, 32, and 42 week-old Pcyt2+/− mice and littermate controls. Plasma LPL was inhibited with P407 injection, and VLDL-TAG was determined in 0–4 h intervals. At 8 weeks of age, VLDL-TAG secretion was similar in Pcyt2+/− mice and control littermates. A modest but significant increase in VLDL secretion was present in 32-week-old Pcyt2+/− mice; and 42-week-old Pcyt2+/− mice showed significantly elevated VLDL secretion compared to littermates. The level of activity (B) and expression (C) of the liver MTP in 8, 32, and 42 week-old mice. MTP activity was expressed as fluorescence (F) U.min−1.μg of protein−1 for n = 5 mice in each group. Statistical significance (* and **) at each point was determined by Student's t-test at P < 0.05.
Postprandial TAG turnover is reduced in Pcyt2+/− mice
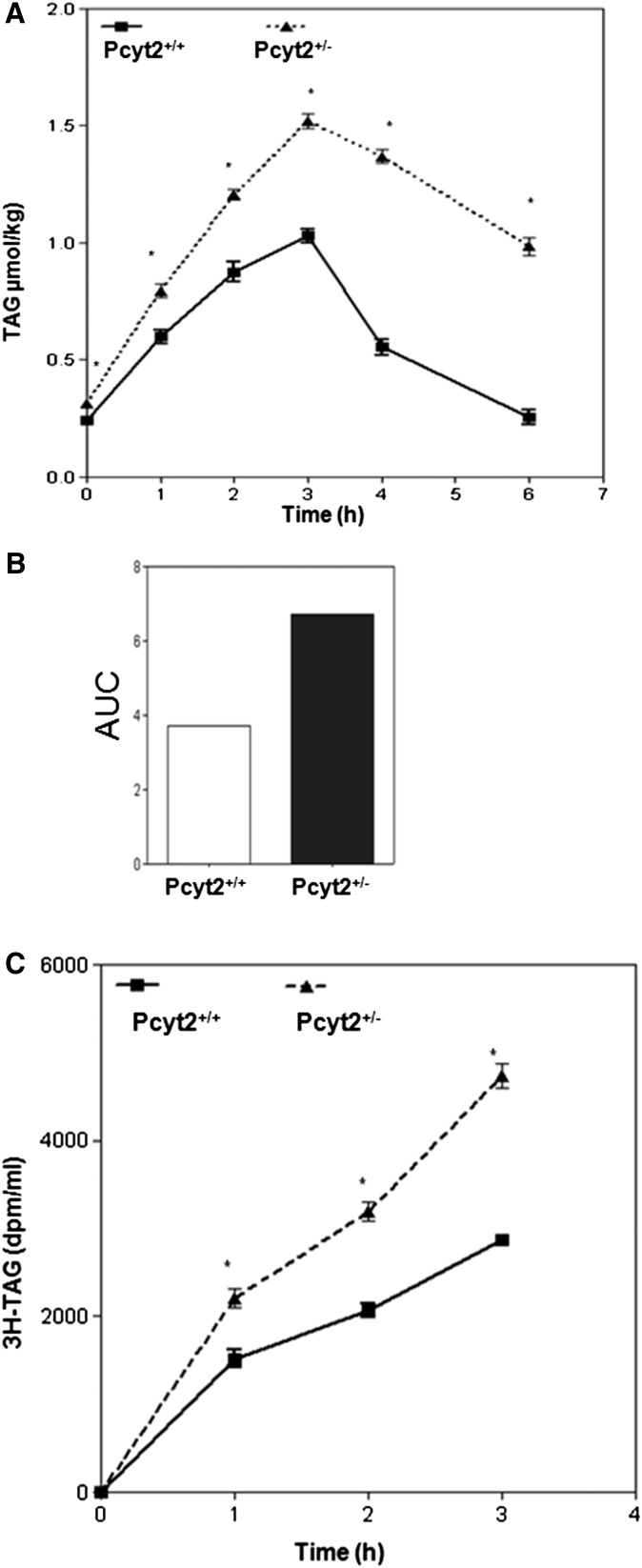
We next determined whether the Pcyt2+/− mice had any defects in the absorption and processing of postprandial lipids. TAG content in the plasma was followed after animals were given an intragastric bolus of olive oil, as shown in Fig. 2A, B. Pcyt2-deficient mice exhibited a faster appearance of TAG in the plasma, and this was maintained at levels higher and longer than in control littermates. Based on the AUC, the quantitative difference in the postprandial TAG responses between deficient and control animals was 40% (Fig. 2B), showing that the Pcyt2+/− mice had both an increased intestinal secretion and a delayed TAG clearance from the plasma, and those two aspects of Pcyt2+/− hypertriglyceridemia were separately examined using a radiolabeled TAG substrate ([3H]TO) as described below.
Fig. 2.
Pcyt2 deficiency modifies postprandial lipid response. Plasma TAG turnover is reduced (A). Pcyt2+/− and littermate (Pcyt2+/+) control mice were given an intragastric bolus of 200 µl of olive oil, and a blood sample was drawn at 0, 1, 2, 3, 4, and 6 h after the lipid load. Total plasma lipid content was expressed as TAG (µmol/kg) ± SD for four mice per group. Shown in B is the integrated AUC of data in A for both wild-type and knockout animals. C: Intestinal TAG secretion is increased. Pcyt2+/− and littermate controls were injected intravenously with 500 mg/kg of 10% P407 LPL inhibitor and were immediately given an intragastric load of [3H]TO in 200 µl of olive oil as in A. Blood samples were drawn at 0, 1, 2, and 3 h, and total lipids were extracted from plasma. Shown is the [3H] radioactivity in TAG fraction for four mice in each group. Statistical significance (*) at each point was determined by Student's t-test at P < 0.05.
Intestinal TAG secretion is elevated in Pcyt2+/− mice
To investigate the extent to which intestinal secretion may contribute to Pcyt2+/− hypertriglyceridemia, the animals were intravenously injected with the lipase inhibitor P407 as above and then given an intragastric load of labeled olive oil. The secretion of intestinal lipids was monitored by the appearance of 3H-related activity in the plasma TAG at various times after the lipid load. As shown in Fig. 2C, both groups of animals displayed a continuous appearance of the radiolabeled TAG in the plasma when measurements were performed for 3 h after lipid load; however, more [3H] activity appeared in the plasma of Pcyt2+/− animals compared with control littermates. This was a strong indication that Pcyt2-deficient intestinal epithelia had acquired an accelerated secretion of the postprandial lipids.
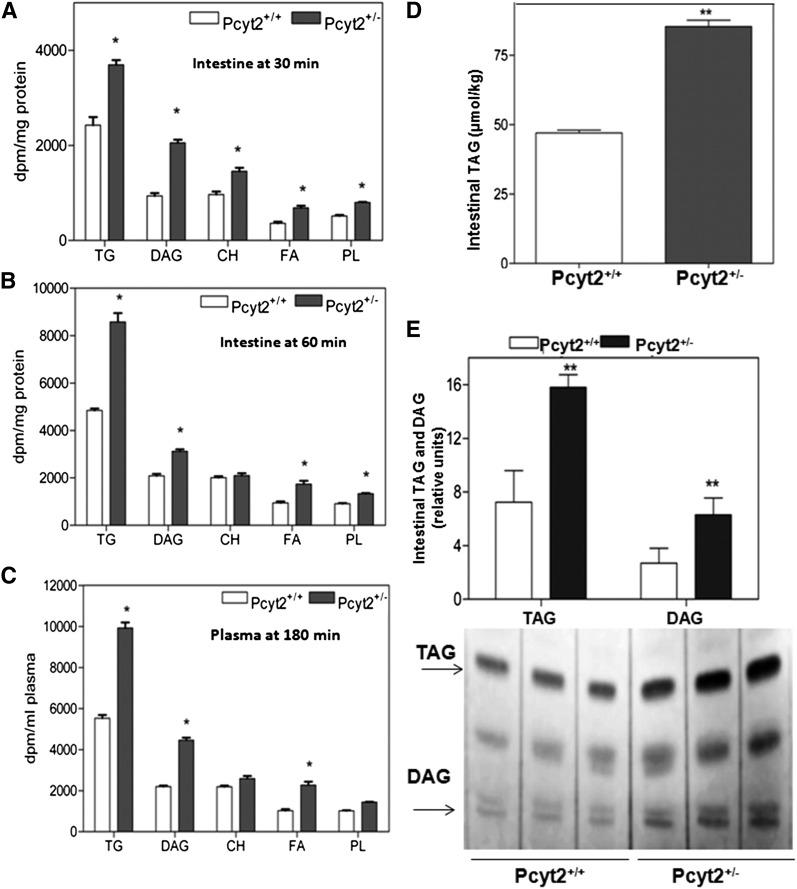
Postprandial lipids in intestinal mucosa and plasma were investigated in the presence of the lipase inhibitor to prevent plasma degradation, as described above. As expected, most radiolabeled lipids were in the form of [3H]TAG in both types of animals (Fig. 3A–C). After 1 h of lipid load, Pcyt2+/− intestinal mucosa had elevated (40%) radioactivity in TAG, DAG, and FFA fractions and 20–30% increase in other lipids (Fig. 3B). In 3 h plasma, the [3H]TAG activity was similar to the activities in the intestinal mucosa, containing ∼40% more label in Pcyt2+/− plasma than in the wild-type plasma. Postprandial [3H]DAG and [3H]FFAs were 2-fold higher in the Pcyt2+/− plasma compared with wild-type (Fig. 3C). A 2–3-fold ratio between [3H]TAG and [3H]DAG was associated with all measurements shown in Fig. 3A–C. We showed previously that the major underlying mechanism of disease progression in Pcyt2+/− mice was a shift in lipid and energy metabolism to remove excess DAG and TAG unused in the PE–Kennedy pathway. This results in elevated lipogenesis and TAG synthesis even early in development and causes age-related accumulation of lipids in adipocytes, liver, muscle, and perhaps other organs (8, 16, 17). The actual TAG content (Fig. 3D) and the relative change in TAG and DAG (Fig. 3E) were 1.8–2.3-fold higher in the intestinal mucosa of Pcyt2+/− mice compared with littermate controls, demonstrating that in addition to accumulation and increased secretion of hepatic lipids in postabsorptive states, Pcyt2+/− mice also have increased intestinal lipids and secretion in postprandial states.
Fig. 3.
Differences in intestinal lipids and secretion. A: Intestinal profiles of TAG, DAG, FFAs (FA), total cholesterol (CH), and total phospholipids (PL) 30 min and (B) 60 min after the [3H]TO-containing fat load. C: Plasma lipid profiles 180 min after the [3H]TO lipid load. Pcyt2+/− and Pcyt2+/+ (n = 5). The values shown in A and B are dpm/mg of protein for intestinal mucosa and in C are dpm/ml for mouse plasma at P < 0.05. D: Intestinal mucosa TAG (µmol/kg) is elevated 1.8-fold in Pcyt2+/− mice relative to the wild-type mice (40 wk-old; n = 4 and P < 0.0001). E: Iodine-stained silica gel showing relative increases in DAG and TAG levels (2.3-fold and 1.9-fold, respectively) in the samples as in D.
Plasma clearance of TAG-rich particles is impaired in Pcyt2+/− mice
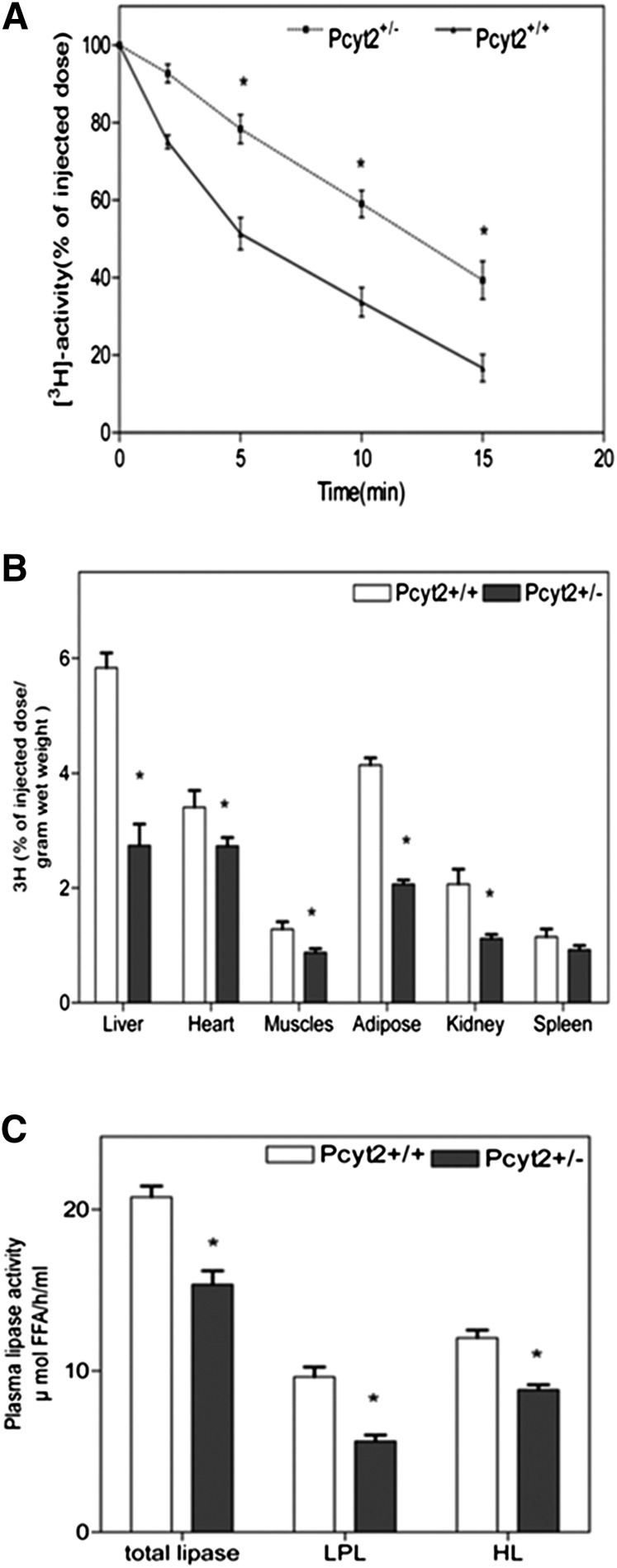
To establish whether impaired degradation of plasma TAG also contributed to the Pcyt2+/− hypertriglyceridemia, animals were injected with labeled TAG-rich lipid particles, and the particle disappearance from circulation was monitored. As evinced from the relative radioactivity remaining in the plasma, shown in Fig. 4A, the rate of disappearance of the [3H]TO-labeled lipids was markedly slower in the Pcyt2+/− mice (the half-life, t1/2 = 43.30 min−1) than in the littermate controls (the half-life, t1/2 = 5.17 min−1), indicating inefficient hydrolysis of both VLDL and CM in the heterozygous mice.
Fig. 4.
Pcyt2 deficiency inhibits plasma lipid clearance. A: Clearance of TAG-rich lipid particles is reduced in Pcyt2+/− plasma. [3H]TO (100 µg of total TAG) was injected in Pcyt2+/− and littermate controls via the inferior vena cava, and blood samples were taken at various times (2.5–15 min) postinjection. Plasma radioactivity (dpm) was determined and expressed as a percent of the injected dose vs. time (min); n = 4 in each group. B: Delivery of TAG-rich lipid particles is reduced in multiple Pcyt2+/− tissues. [3H]TO particles (100 µg of TAG) were injected as in A, and radioactivity (dpm) was determined at 30 min in homogenized tissues (liver, heart, muscle, adipose, kidney, and spleen). Data are shown as a percent of the injected dose/g weight for four animals; *P < 0.05. C: Pcyt2+/− mice have impaired plasma HL and LPL activities. Fasted Pcyt2+/− and wild-type mice were injected via retro-orbital plexus with heparin (0.1 U/g), and blood was collected 20 min after injection. Plasma was incubated for 30 min at 37°C with a [3H]TO in the absence or presence of 1 M NaCl to establish both the LPL and HL activity. Generated [3H] oleate was extracted, and activity was determined as [oleate] µmol/h/g tissue ± SD (n = 4) at *P < 0.05.
Total lipid uptake in various tissues was examined 30 min after the [3H]TAG particle injection (Fig. 4B). The associated tissue radioactivity was 50–60% lower in Pcyt2+/− liver, total adipose tissue, and kidney and significantly reduced in total skeletal muscle and heart relative to control tissue homogenates. Taken together, these data indicate that Pcyt2+/− mice acquire an impaired clearance of TAG-rich particles from the plasma; therefore, we next investigated lipase activity in Pcyt2-deficient tissues.
Plasma LPL and HL activities are reduced in Pcyt2+/− mice
Reduced postprandial TAG turnover (Fig. 2A, B) and delayed TAG particle clearance (Fig. 4A) from Pcyt2+/− circulation were both consistent with decreased plasma lipolysis, mainly controlled by the plasma LPL and HL activities. To investigate this, lipase activities were measured in the postheparin plasma of both genotypes. As shown in Fig. 4C, the total plasma lipase (33%) and individual LPL (44%) and HL (27%) activities were significantly reduced in the Pcyt2+/− plasma compared with control plasma.
Altered expression of LPL and angiopoietin-like protein 4
We next investigated whether decreased tissue LPL availability may contribute to the reduced activity in Pcyt2+/− mice. LPL is mainly expressed in adipose tissue and to some extent in skeletal muscle and heart. LPL mRNA was completely absent from intestine and unchanged in the total skeletal muscle and liver of Pcyt2+/− mice. On the other hand, Pcyt2+/− adipose tissue and heart homogenates had 1.7- and 1.5-fold less LPL mRNA (Fig. 5A, B). Angptl4 is a very potent inhibitor of LPL activity and a stimulator of adipose tissue lipolysis (31), and, as shown in Fig. 5C, D, there is a marked 1.97-fold increase in the expression of serum Angptl4 in Pcyt2+/− mice relative to control littermates. Therefore, both reduced LPL tissue expression and increased LPL inhibition contributed to the reduced LPL activity in the Pcyt2+/− plasma.
Fig. 5.
Pcyt2+/− mice have reduced tissue LPL and increased plasma Angptl4. A: PCR results for LPL mRNA expression in various tissues of Pcyt2+/− and littermate control mice (n = 4). B: LPL mRNA levels relative to control G3PDH showing significantly reduced LPL expression in homogenates of Pcyt2+/− heart (1.5-fold, P < 0.002) and adipose (1.74-fold, P < 0.0006) and no change in skeletal muscle and liver total homogenates. Immunoblots (C) and densitometric analysis (D) showing increased amounts of Angptl4 protein in Pcyt2+/− plasma relative to control plasma (1.97-fold, P < 0.0007, n = 4).
Plasma apoB and intestinal MTP activity are elevated in Pcyt2+/− mice
The lipid tolerance test (Fig. 2A) established a significant postprandial contribution to plasma Pcyt2+/− TAG, therefore the postprandial apoB lipoprotein content was also determined. As shown in Fig. 6A–C, apoB-100 and apoB-48 content was dramatically increased, with apoB-48 levels 5-fold and apoB-100 levels 1.8-fold higher than in the control plasma. The elevation in plasma apoB proteins is consistent with the increased VLDL secretion (Fig. 1A) and with the previously established accumulation of the VLDL particles in Pcyt2+/− plasma (16). To confirm that intestinal lipoprotein production was elevated in Pcyt2 deficiency, we also measured the intestinal MTP activity in 42 week-old mice. As shown in Fig. 6D, the MTP activity was elevated 3-fold, demonstrating that Pcyt2+/− mice also produced more lipoprotein (chylomicron) particles in the small intestine.
Fig. 6.
Pcyt2+/− mice have increased plasma apoB and intestinal MTP activity. A: Immunoblotting of Pcyt2+/− and control plasma for apoB-48 and apoB-100 3 h after an intragastric load of 200 µl of olive oil; β tubulin was used as a loading control. B,C: ApoB-48 and apoB-100 were dramatically elevated in Pcyt2+/− plasma. D: MTP activity was upregulated in Pcyt2+/− small intestine and was expressed as fluorescence U/min/μg protein (n = 5 and P < 0.05).
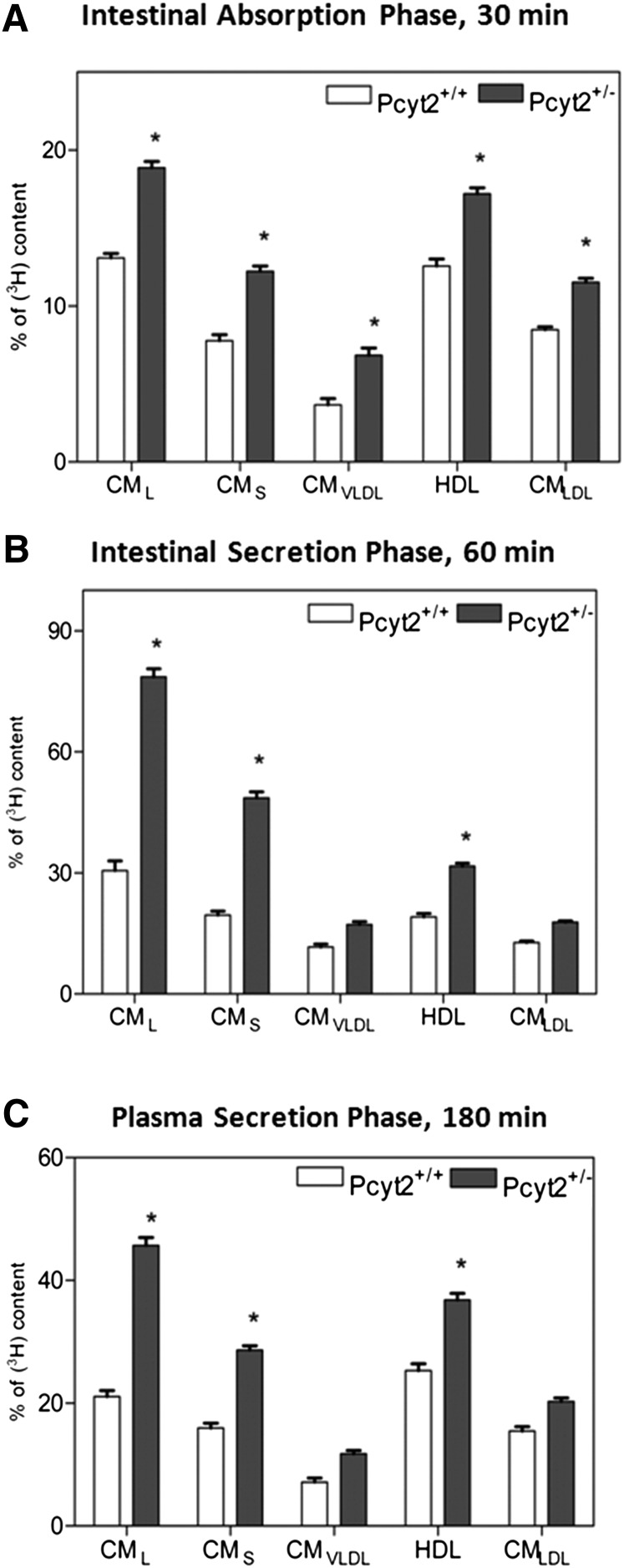
Chylomicron production and secretion are upregulated in Pcyt2+/minus mice
To gain further insight into the mechanism of elevated postprandial TAG and apoB containing lipoproteins in Pcyt2+/− plasma, we next examined the postprandial profiles of lipoproteins secreted in vitro from isolated enterocytes and in vivo from intestine (Fig. 7). We conducted a density lipoprotein centrifugation to separate various lipoproteins and to correlate the extent of lipidation within different fractions from the primary enterocytes (Fig. 7A), those secreted into the media (Fig. 7B), and those secreted in vivo (Fig. 7C). For both genotypes, the absorbed lipids were associated with similar lipoprotein fractions and were in a descending order from CML=CMLDL>CMS=HDL>CMVLDL. However, the enterocytes isolated from Pcyt2+/− intestine contained proportionally 30–50% more lipids compared with control cells in all lipoprotein fractions (Fig. 7A). This directly demonstrates that the intestinal absorption was significantly increased in the Pcyt2+/− mice. To investigate the secretion phase, the isolated enterocytes were incubated for 3 h, and the lipoproteins secreted into media were examined. The type and the order of the secreted lipoproteins was similar in both genotypes, and it was dominated by large and small CM fractions, CML>CMS>CMLDL>HDL = CMVLDL. The most-abundant fractions, CML and CMS, had >2-fold more lipids in the Pcyt2+/− enterocyte media than the CML and CMS fractions isolated from the control media (Fig. 7B). Finally, the plasma lipoproteins secreted in vivo were fractionated (Fig. 7C). The extent of lipidation of CM fractions was similar to those observed for enterocyte media, and Pcyt2+/− plasma CML and CMS fractions again contained >2-fold more lipids than the CML and CMS from the control plasma. Not surprisingly, plasma CMLDL and HDL fractions were more abundant than those obtained in the enterocyte media, but the lipid content was similar in vitro and in vivo, and it was higher in the CMLDL and HDL-like fractions isolated from the Pcyt2+/− than in the wild-type plasma.
Fig. 7.
Differences in lipidation of intestinally derived lipoproteins. A: Density profile of enterocyte lipoproteins immediately (30 min) after an intragastric load of [3H]TO. B: The enterocyte lipoproteins secreted 3 h after the [3H]TO load. C: Plasma lipoprotein profiles 1 h after the lipid load; values are shown for n = 5 and as percent of dpm/mg protein for enterocyte in A, B and percent dpm/ml for plasma in C. Statistical significance (*) between the two genotypes was determined by Student's t-test at P < 0.05.
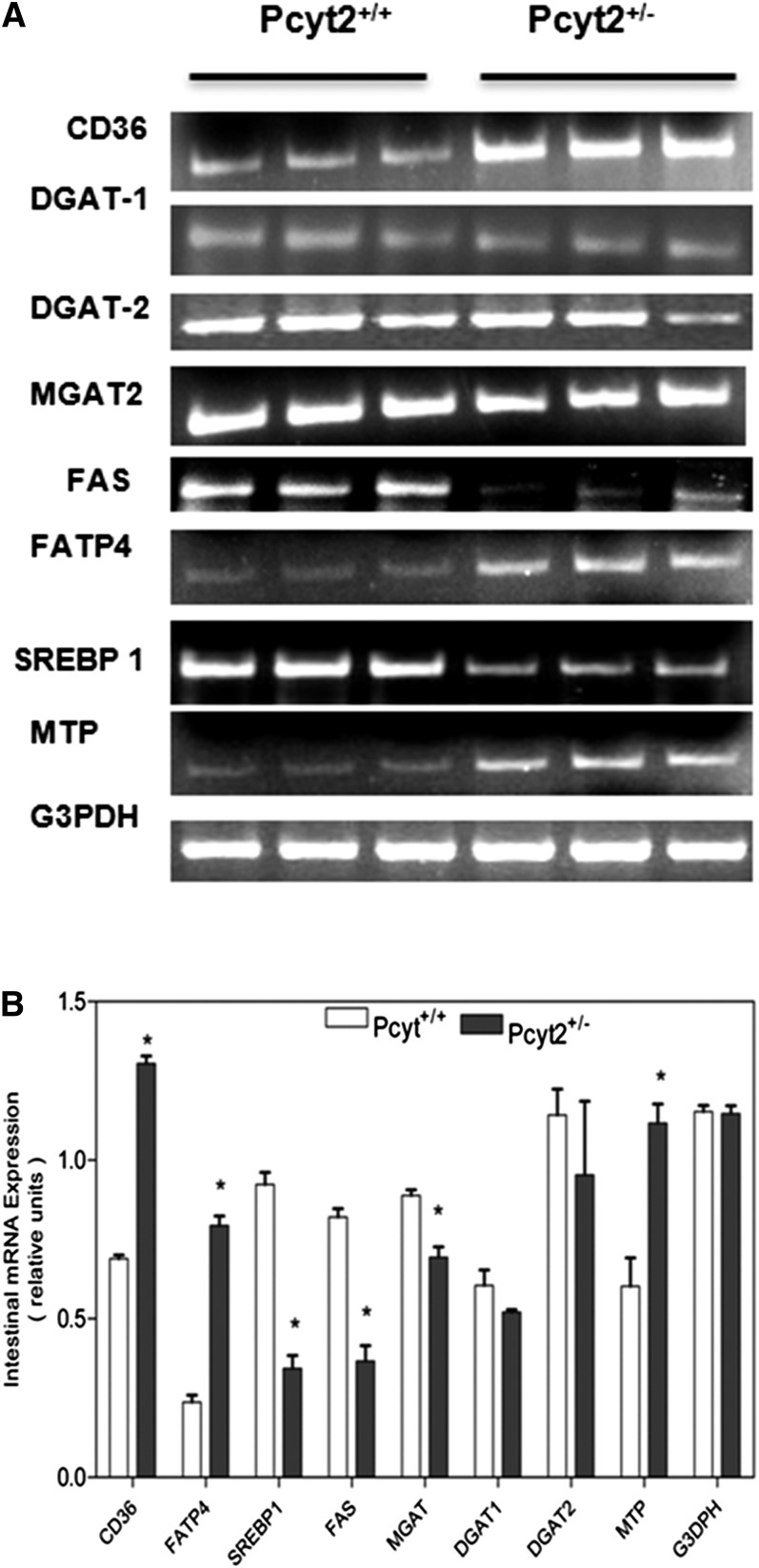
Pcyt2+/− intestinal genes for FA absorption and chylomicron formation are upregulated
To further determine the mechanism for the elevated Pcyt2+/− intestinal lipid absorption and chylomicron secretion, the genes associated with FA transport, TAG synthesis, and chylomicron assembly were examined in both genotypes, as shown in Fig. 8. We found that postprandial mRNA expression of FA transport protein 4 (FATP4), the only FATP in the intestine, was 3-fold higher and that the main enterocyte FA transporter, CD36, was 2-fold higher in Pcyt2+/− small intestine than in control littermates. The expression of MTP, which has a critical role in chylomicron assembly, was also 2-fold higher in Pcyt2+/− intestine. Interestingly, the genes involved in intracellular TAG formation, DGAT1, and DGAT2 did not change significantly, and they probably are more regulated by posttranslational mechanisms such as phosphorylation (32). FA synthesis normally is not a major event in this tissue, but could potentially be modified in insulin resistance and diabetes (32, 33). The postprandial expression of the main lipogenic genes SREBP1 and FAS were dramatically reduced (3-fold and 2.8-fold, respectively) showing that enterocyte de novo synthesis of FA was reduced in Pcyt2+/− mice. Therefore, the FA pool available for the increased TAG formation and chylomicron assembly in Pcyt2+/− intestine was produced by increased uptake and intracellular transport of dietary FAs (33).
Fig. 8.
Intestinal genes for lipid transport and chylomicron formation are overexpressed in Pcyt2 deficiency. A: Representative RT-PCR expression data for the FA synthase (FAS), diacylglycerol transferase 1 and 2 (DGAT-1 and -2), monoacylglycerol acyltransferase (MGAT2), FATP4, FA transporter CD36, sterol-regulatory binding protein 1 (SREBP1), microsomal transfer protein (MTP), and glycerol-3-phosphate dehydrogenase (G3PDH) loading control in Pcyt2+/− and Pcyt2+/+ small intestine. B: Average mRNA band densities from at least three separate RNA isolations in each group of animals. Statistical significance (*) between the two genotypes was determined by Student's t-test at P < 0.05.
DISCUSSION
Pcyt2 regulates de novo PE phospholipid synthesis, an anabolic pathway that utilizes ATP and DAG. The attenuation of this pathway in Pcyt2+/− mice creates a surplus of those metabolites and reduces demands for energy production by mitochondrial FA oxidation. Indeed, young Pcyt2+/− mice experience reduced weight loss after prolonged fasting and have upregulated liver lipogenesis prior to development of obesity, insulin resistance, and hyperlipidemia (16). Therefore, the redistribution of DAG/FAs from membrane PE toward TAG creates a state of positive energy balance in Pcyt2 deficiency. These inherent changes, in combination with the age-related decline in metabolic efficiency (34, 35) and changes in energy and nutrient signaling networks (36, 37) are probably most responsible for the disease progression in this model.
In the present study, we focused on Pcyt2+/− hyperlipidemia that developed in older animals. We established that Pcyt2+/− hyperlipidemia was a net result of increased secretion of TAG-rich lipoproteins and reduced capacity for lipid clearance from the plasma. Furthermore, there was a strong relationship between Pcyt2 deficiency and the activity and expression of genes involved in plasma lipolysis and intestinal lipid absorption and secretion, uncovering for the first time that Pcyt2 has an intrinsic role in the regulation of liver lipid secretion, plasma lipolysis, and postprandial lipid metabolism.
Initially, we reported that older (32 week) Pcyt2+/− mice have upregulated liver lipogenic genes, reduced FA oxidation, and increased FFA uptake, and accumulate liver lipids and have characteristic lipoprotein profiles with elevated VLDL content and normal HDL and LDL content (16). Here, we have established that the liver VLDL-TAG secretion is not impaired in young animals, but becomes significantly elevated in older (32 week and 42 week) Pcyt2+/− mice. Furthermore, we provide several lines of evidence that in addition to being hypertriglyceridemic, Pcyt2+/− mice additionally exhibit an increased capacity to process exogenous lipids in intestinal epithelial cells and have reduced capacity to clear postprandial lipids from circulation. Using an oral lipid tolerance test, we demonstrated that Pcyt2+/− mice had significantly reduced turnover of circulating TAG compared with wild-type littermates. By separate examination of plasma TAG influx and degradation, we established that Pcyt2+/− mice increase the processing of dietary lipids, as well as reduce plasma lipolysis, which together resulted in a longer TAG half-life in the plasma. We demonstrate that postprandial lipids appeared in Pcyt2+/− circulation at rates faster than in the control littermates. Lipoprotein density fractionation established that Pcyt2+/− enterocytes and plasma had substantially increased lipidation of chylomicron particles, and that increased chylomicron production in Pcyt2 deficiency was accompanied by an abundant presence of apoB-48 in the plasma. Finally, the facilitated processing of dietary lipids in Pcyt2+/− intestine was supported by elevated expression and/or activity of intestinal genes responsible for FA transport and esterification (CD36 and FATP4 expression) and chylomicron assembly and secretion (increased MTP gene expression and activity). An additional possibility for the facilitation of intestinal lipid uptake may be that digested products (FFA, DAG, and MAG) also experience a lower barrier to absorption, linked to modified membrane fluidity and composition. Pcyt2+/− mice have normal membrane PC/PE and cholesterol/phospholipid ratios; however, the elevated saturated and monounsaturated FAs and reduced content of PUFAs in the membrane PE (8) may be significant. Whether this uniquely modified PE content modifies membrane lipid absorption remains unknown.
In addition to pronounced lipogenesis and reduced FA oxidation, older Pcyt2+/− mice also have increased uptake of circulating FFAs, the processes we initially found to be responsible for development of hepatic steatosis (16). The elevated expression of lipogenic genes SREBP1 and FAS in the Pcyt2+/− liver cells could be normalized by fully restoring the Pcyt2 expression and function (17). Interestingly, intestinal lipogenesis was reduced in Pcyt2+/− mice, as was shown by lower expression of the key lipogenic genes SREBP1 and FAS, further indicating that excess FAs absorbed from diet (33), not synthesized de novo from glucose, constituted the bulk of the intracellular FA pool used for the chylomicron formation. Therefore, it is apparent that Pcyt2 deficiency differently influenced FA and glucose metabolism in liver and intestine and that the metabolic disturbances in multiple organs contributed to the development of the Pcyt2+/− mice hyperlipidemia.
The abnormalities in lipoprotein turnover were evident from reduced plasma lipid degradation and from reduced lipid uptake in multiple Pcyt2+/− tissues. The extended half-life of circulating TAG was accompanied by a 50% reduction in liver and adipose tissue uptake in Pcyt2+/−mice. These data agree with our previous report, suggesting unchanged liver TAG degradation by lipolysis (16), and also that receptor-mediated lipoprotein uptake could be impaired in the Pcyt2+/− liver and adipocytes. The impaired catabolism of plasma TAG in Pcyt2+/− mice was also a result of reduced postheparin LPL and HL activities. The molecular mechanisms for the reduced activities of LPL and HL in Pcyt2+/− mice implicate factors other than the product inhibition by FFAs, because we previously established that Pcyt2-deficient animals had only mildly elevated FFA content in the plasma (16). We showed that downregulation of plasma LPL in Pcyt2+/− mice was caused by reduced LPL mRNA in adipose tissue and heart, and we identified that the potent LPL inhibitor Angptl4 was abundant in Pcyt2+/− plasma. These changes probably contribute to the reduced LPL activity and Pcyt2+/− hyperlipidemia, but other factors, such as apoC-III and the LPL transport protein glycosyl phosphatydylinositol-anchored HDL binding protein 1 are likely to have additionally altered the LPL content and activity (38–40).
HL is released from the liver, and the HL activity in the plasma is predominantly regulated by the HDL content and composition. HL can also control the VLDL TAG pool in both humans and mice (41, 42). The HL activity was significantly reduced in Pcyt2+/− plasma, implicating additional complications in HDL secretion and/or plasma composition in Pcyt2+/− mice. Plasma TAG FA composition is significantly modified in heterozygous mice, containing elevated content of C16 and C18 n-6 FAs (16), probably the products of the elevated Pcyt2+/− mice intestinal absorption, but also increased liver VLDL secretion and lipogenesis (16, 17). Future studies on the regulation of the specific contributions of liver and intestinal lipoproteins and lipoprotein receptors in Pcyt2 deficiency will help in better understanding the mechanisms driving the impaired plasma lipid clearance and how lipases and regulatory proteins contribute to the accumulation of plasma TAG in Pcyt2 deficiency.
The disruption of Pcyt2 is responsible not only for altering TAG synthesis, but also for affecting other aspects of lipid homeostasis, such as FA oxidation and reduced PE availability as a source of FA (16, 17). Pcyt2+/− mice also develop insulin resistance, even on a chow diet (16), which, together with the elevated postprandial lipids (also on a chow diet), imply that in mice chronically exposed to a high-fat diet regimen, reduced Pcyt2 activity might also contribute a high-fat diet-induced hypertriglyceridemia and development of type-2 diabetes, as seen in humans. Based on our initial work discussed earlier and information from numerous diet-induced obesity models, we anticipate that the phenotype described here for the older Pcyt2+/− mice would appear earlier in the life if young mice were fed a high-fat diet. Postprandial lipemia is a well-known risk factor for development of type-2 diabetes (43), and available data suggest that the expression of human Pcyt2 is reduced in adipose tissue in women with polycystic ovary syndrome (44), in myotubes from patients with type-2 diabetes (45), and in MODY type-2 diabetes (46).
In conclusion, we establish that hypertriglyceridemia observed in the Pcyt2-heterozygous mouse can be attributed to elevated lipid secretion and over-production of TAG-rich particles. This, in combination with impaired lipolytic conversion of TAG-rich remnants, results in subsequent reduced delivery of TAG-derived lipids to peripheral tissues. The heterozygous disruption of Pcyt2 results in aberrant lipid absorption and the age-related development of obesity and metabolic syndrome, which opens an interesting new area to be considered when studying lipid-related disorders, impairments in membrane phospholipid gene function, and phospholipid homeostasis.
Footnotes
Abbreviations:
- AUC
- area under the curve
- CM
- chylomicron
- DAG
- diacylglycerol
- ER
- endoplasmic reticulum
- FATP
- FA transport protein
- LSC
- liquid scintillation counting
- MTP
- microsomal triglyceride transfer protein
- P407
- poloxamer 407
- PC
- phosphatidylcholine
- Pcyt1
- CTP:phosphocholine cytidylyltransferase
- Pcyt2
- CTP-phosphoethanolamine cytidylyltransferase
- PE
- phosphatidylethanolamine
- PEMT
- phosphatidylethanolamine methylation
- PS
- phosphatidylserine
- TAG
- triglyceride
- TO
- triolein
This work was supported by grants from the Canadian Institutes of Health Research-CIHR (MOP 86448) (M.B.) and NSERC Discovery (D.V.). We declare that CIHR had no involvement in the collection and data analysis, in the interpretation and writing of the manuscript, and in the decision to submit the manuscript for publication.
REFERENCES
- 1.Guo Y., Walther T. C., Rao M., Stuurman N., Goshima G., Terayama K., Wong J. S., Vale R. D., Walter P., Farese R. V. 2008. Functional genomic screen reveals genes involved in lipid-droplet formation and utilization. Nature. 453: 657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Igal R. A., Caviglia J. M., De Gomez Dumm I. N., Coleman R. A. 2001. Diacylglycerol generated in CHO cell plasma membrane by phospholipase C is used for triacylglycerol synthesis. J. Lipid Res. 42: 88–95. [PubMed] [Google Scholar]
- 3.Caviglia J. M., De Gomez Dumm I. N., Coleman R. A., Igal R. A. 2004. Phosphatidylcholine deficiency upregulates enzymes of triacylglycerol metabolism in CHO cells. J. Lipid Res. 45: 1500–1509. [DOI] [PubMed] [Google Scholar]
- 4.Vance D. E. 2008. Role of phosphatidylcholine biosynthesis in the regulation of lipoprotein homeostasis. Curr. Opin. Lipidol. 19: 229–234. [DOI] [PubMed] [Google Scholar]
- 5.Vance D. E., Vance J. E. 2009. Physiological consequences of disruption of mammalian phospholipid biosynthetic genes. J. Lipid Res. 50 (Suppl.): S132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z., Agellon L. B., Allen T. M., Umeda M., Jewell L., Mason A., Vance D. E. 2006. The ratio of phosphatidylcholine to phosphatidylethanolamine influences membrane integrity and steatohepatitis. Cell Metab. 3: 321–331. [DOI] [PubMed] [Google Scholar]
- 7.Fu S., Yang L., Li P., Hofmann O., Dicker L., Hide W., Lin X., Watkins S. M., Ivanov A. R., Hotamisligil G. S. 2011. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 473: 528–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fullerton M. D., Hakimuddin F., Bakovic M. 2007. Developmental and metabolic effects of disruption of the mouse CTP:phosphoethanolamine cytidylyltransferase gene (Pcyt2). Mol. Cell. Biol. 27: 3327–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bakovic M., Fullerton M. D., Michel V. 2007. Metabolic and molecular aspects of ethanolamine phospholipid biosynthesis: the role of CTP:phosphoethanolamine cytidylyltransferase (Pcyt2). Biochem. Cell Biol. 85: 283–300. [DOI] [PubMed] [Google Scholar]
- 10.Hermansson M., Hokynar K., Somerharju P. 2011. Mechanisms of glycerophospholipid homeostasis in mammalian cells. Prog. Lipid Res. 50: 240–257. [DOI] [PubMed] [Google Scholar]
- 11.Poloumienko A., Cote A., Tie A. Q., Zhu L., Bakovic M. 2004. Genomic organization and differential splicing of the mouse and human Pcyt2 genes. Gene. 325: 145–155. [DOI] [PubMed] [Google Scholar]
- 12.Tie A., Bakovic M. 2007. Alternative splicing of CTP:phosphoethanolamine cytidylyltransferase produces two isoforms that differ in catalytic properties. J. Lipid Res. 48: 2172–2181. [DOI] [PubMed] [Google Scholar]
- 13.Johnson C. M., Yuan Z., Bakovic M. 2005. Characterization of transcription factors and cis-acting elements that regulate human CTP:phosphoethanolamine cytidylyltransferase (Pcyt2). Biochim. Biophys. Acta. 1735: 230–235. [DOI] [PubMed] [Google Scholar]
- 14.Zhu L., Michel V., Bakovic M. 2009. Regulation of the mouse CTP:phosphoethanolamine cytidylyltransferase gene Pcyt2 during myogenesis. Gene. 447: 51–59. [DOI] [PubMed] [Google Scholar]
- 15.Zhu L., Bakovic M. 2008. Liver X receptor agonists inhibit the phospholipid regulatory gene CTP:phosphoethanolamine cytidylyltransferase-Pcyt2. Biochem. Res. Int. 2008: 801849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fullerton M. D., Hakimuddin F., Bonen A., Bakovic M. 2009. The development of a metabolic disease phenotype in CTP: phosphoethanolamine cytidylyltransferase deficient mice. J. Biol. Chem. 284: 25704–25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fullerton M., Bakovic M. 2010. Complementation of the metabolic defect in CTP: phosphoethanolamine cytidylyltransferase (Pcyt2) deficient primary hepatocites. Metabolism. 59: 1691–1700. [DOI] [PubMed] [Google Scholar]
- 18.Leonardi R., Frank M. W., Jackson P. D., Rock C. O., Jackowski S. 2009. Elimination of the CDP-ethanolamine pathway disrupts hepatic lipid homeostasis. J. Biol. Chem. 284: 27077–27089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millar J. S., Cromley D. A., McCoy M. G., Rader D. J., Billheimer J. T. 2005. Determining hepatic triglyceride production in mice: comparison of poloxamer 407 with Triton WR-1339. J. Lipid Res. 46: 2023–2028. [DOI] [PubMed] [Google Scholar]
- 20.Johnston T. P. 2010. Poloxamer 407 as a general lipase inhibitor: its implication in lipid metabolism and atheroma formation in C57BL/6 mice. J. Pharm. Pharmacol. 62: 1807–1812. [DOI] [PubMed] [Google Scholar]
- 21.Bligh E. G., Dyer W. J. 1959. A rapid method of total lipid extraction and purification. Can. J. Med. Sci. 37: 911–917. [DOI] [PubMed] [Google Scholar]
- 22.Goudriaan J. R., Santo S. M. S. E., Voshol P. J., Rensen P. C. 2004. The VLDL receptor plays a major role in chylomicron metabolism by enhancing LPL-mediated triglyceride hydrolysis. J. Lipid Res. 45: 1475–1481. [DOI] [PubMed] [Google Scholar]
- 23.Moen C. J., Tholens A. P., Voshol P. J., Rensen P. C. 2007. The Hyplip2 locus causes hypertriglyceridemia by decreased clearance of triglycerides. J. Lipid Res. 48: 2182–2192. [DOI] [PubMed] [Google Scholar]
- 24.Vine D. F., Takechi R., Russell J. C., Proctor S. D. 2007. Impaired postprandial apolipoprotein-B48 metabolism in the obese, insulin-resistant JCR:LA-cp rat: increased atherogenicity for the metabolic syndrome. Atherosclerosis. 190: 282–290. [DOI] [PubMed] [Google Scholar]
- 25.Swift L. L., Jovanovska A., Kakkad B., Ong D. E. 2005. Microsomal triglyceride transfer protein expression in mouse intestine. Histochem. Cell Biol. 123: 475–482. [DOI] [PubMed] [Google Scholar]
- 26.Cheng D., Iqbal J., Devenny J., Chu C-H., Chen L., Dong J., Seethala R., Keim W. J., Azzara A. V., Lawrence R. M. L., et al. 2008. Acylation of acylglycerols by acyl coenzyme A:diacylglycerol acyltransferase 1 (DGAT1). Functional importance of DGAT1 in the intestinal fat absorption. J. Biol. Chem. 283: 29802–29811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luchoomun J., Hussain M. M. 1999. Assembly and secretion of chylomicrons by differentiated Caco-2 cells. Nascent triglycerides and preformed phospholipids are preferentially used for lipoprotein assembly. J. Biol. Chem. 274: 19565–19572. [DOI] [PubMed] [Google Scholar]
- 28.Nayak N., Harrison E. H., Hussain M. M. 2001. Retinyl ester secretion by intestinal cells: a specific and regulated process dependent on assembly and secretion of chylomicrons. J. Lipid Res. 42: 272–280. [PubMed] [Google Scholar]
- 29.Anwar K., Kayden H. J., Hussain M. M. 2006. Transport of vitamin E by differentiated Caco-2 cells. J. Lipid Res. 47: 1261–1273. [DOI] [PubMed] [Google Scholar]
- 30.Iqbal J., Hussain M. M. 2005. Evidence for multiple complementary pathways for efficient cholesterol absorption in mice. J. Lipid Res. 46: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 31.Lu B., Moser A., Shigenaga J. K., Grunfeld C., Feingold K. R. 2010. The acute phase response stimulates the expression of angiopietin like proten 4. Biochem. Biophys. Res. Commun. 391: 1737–1741. [DOI] [PubMed] [Google Scholar]
- 32.Yen C. L., Stone S. J., Koliwad S., Harris C., Farese R. V., Jr. 2008. DGAT enzymes and triacylglycerol biosynthesis. J. Lipid Res. 49: 2283–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hsieh J., Longuet C., Maida A., Bahrami J., Xu E., Baker C. L., Adeli K. 2009. Glucagon-like peptide-2 increases intestinal lipid absorption and chylomicron production via CD36. Gastroenterology. 137: 997–1005. [DOI] [PubMed] [Google Scholar]
- 34.De Guzman J. M., Ku G., Fahey R., Youm Y. H., Kass I., Ingram D. K., Dixit V. D., Kheterpal I. Chronic caloric restriction partially protects against age-related alteration in serum metabolome. Age (Dordr.). Epub ahead of print. June 4, 2012. DOI 10.1007/s11357-012-9430-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sample J., Cleland J. G., Seymour A. M. 2006. Metabolic remodeling in the aging heart. J. Mol. Cell. Cardiol. 40: 56–63. [DOI] [PubMed] [Google Scholar]
- 36.Ming X. F., Montani J. P., Yang Z. 2012. Perspectives of Targeting mTORC1–S6K1 in cardiovascular aging. Front Physiol. 3: 5–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kapahi P., Chen D., Rogers A. N., Katewa S. D., Li P. W., Thomas E. L., Kockel L. 2010. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metab. 11: 453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichtenstein L., Kersten S. 2010. Modulation of plasma TG lipolysis by angiopoietin-like proteins and GPIHBP1. Biochim. Biophys. Acta. 1801: 415–420. [DOI] [PubMed] [Google Scholar]
- 39.Dallinga-Thie G. M., Franssen R., Mooij H. L., Visser M. E., Hassing H. C., Peelman F., Kastelein J. J., Péterfy M., Nieuwdorp M. 2010. The metabolism of triglyceride-rich lipoproteins revisited: new players, new insight. Atherosclerosis. 211: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies B. S., Beigneux A. P., Fong L. G., Young S. G. 2012. New wrinkles in lipoprotein lipase biology. Curr. Opin. Lipidol. 23: 35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chatterjee C., Sparks D. L. 2011. Hepatic lipase, high density lipoproteins, and hypertriglyceridemia. Am. J. Pathol. 178: 1429–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pratt S. M., Chiu S., Espinal G. M., Shibata N. M., Wong H., Warden C. H. 2010. Mouse hepatic lipase alleles with variable effects on lipoprotein composition and size. J. Lipid Res. 51: 1035–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hsieh J., Hayashi A. A., Webb J., Adeli K. 2008. Postprandial dyslipidemia in insulin resistance: mechanisms and role of intestinal insulin sensitivity. Atheroscler. Suppl. 9: 7–13. [DOI] [PubMed] [Google Scholar]
- 44.Cortón M., Botella-Carretero J. I., Benguría A., Villuendas Zaballos A., San Millán J. L., Escobar-Morreale H. F., Peral B. 2007. Differential gene expression profile in omental adipose tissue in women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 92: 328–337. [DOI] [PubMed] [Google Scholar]
- 45.Frederiksen C. M., Højlund K., Hansen L., Oakeley E. J., Hemmings B., Abdallah B. M., Brusgaard K., Beck-Nielsen H., Gaster M. 2008. Transcriptional profiling of myotubes from patients with type-2 diabetes: no evidence for a primary defect in oxidative phosphorylation genes. Diabetologia. 51: 2068–2077. [DOI] [PubMed] [Google Scholar]
- 46.Lee Y. H., Nair S., Rousseau E., Allison D. B., Page G. P., Tataranni P. A., Bogardus C., Permana P. A. 2005. Microarray profiling of isolated abdominal subcutaneous adipocytes from obese vs non-obese Pima Indians: increased expression of inflammation-related genes. Diabetologia. 48: 1776–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]