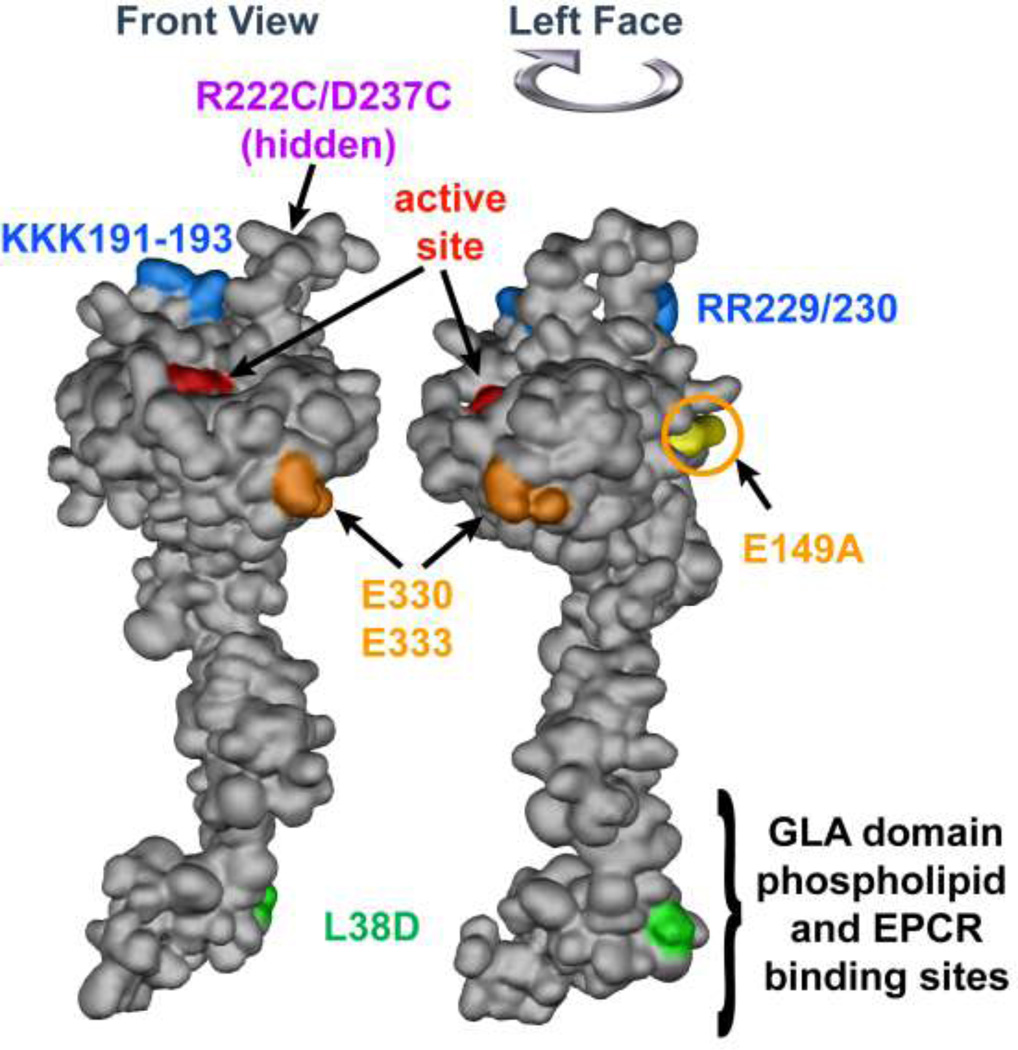
Figure 3. APC space-filling model showing mutations that differentiate amino acid residue requirements for anticoagulant versus cytoprotective activities.
The model of APC extending from bottom to top depicts the N-terminal Gla domain at the bottom which binds EPCR and phospholipids membranes. The protease domain is at at the top, with the EGF-1and EGF-2 domains in the middle section of the model. The "active site" triad of Ser, His and Asp residues is noted in red. Green highlights the L38D mutation that reduces anticoagulant activity due to reduced protein S enhancement. Gold highlights mutations of E330 and E333 to Ala that selectively reduce PAR1 signaling and the E149A mutation in the C-terminus of the light chain that causes loss of cytoprotective anti-inflammatory and anti-apoptotic activities but gain-of-function of anticoagulant activity due to enhanced protein S cofactor effects. On the top of the model, blue highlights five basic residues (KKK191–193 and RR229/230) which form a large positively charged exosite that recognizes factor Va. When these five basic residues are mutated to Ala, < 10 % anticoagulant activity remains whereas cytoprotective activities remain intact. Purple highlights two residues (R222 and D237 that are not in view) which when mutated to Cys can form a disulfide bond, causing loss of most anticoagulant activity but retention of cytoprotective activity. The model of full length APC [172] is based on the serine protease domain structure of APC (Protein Data Bank entry 1AUT [155]).