Figure 4.
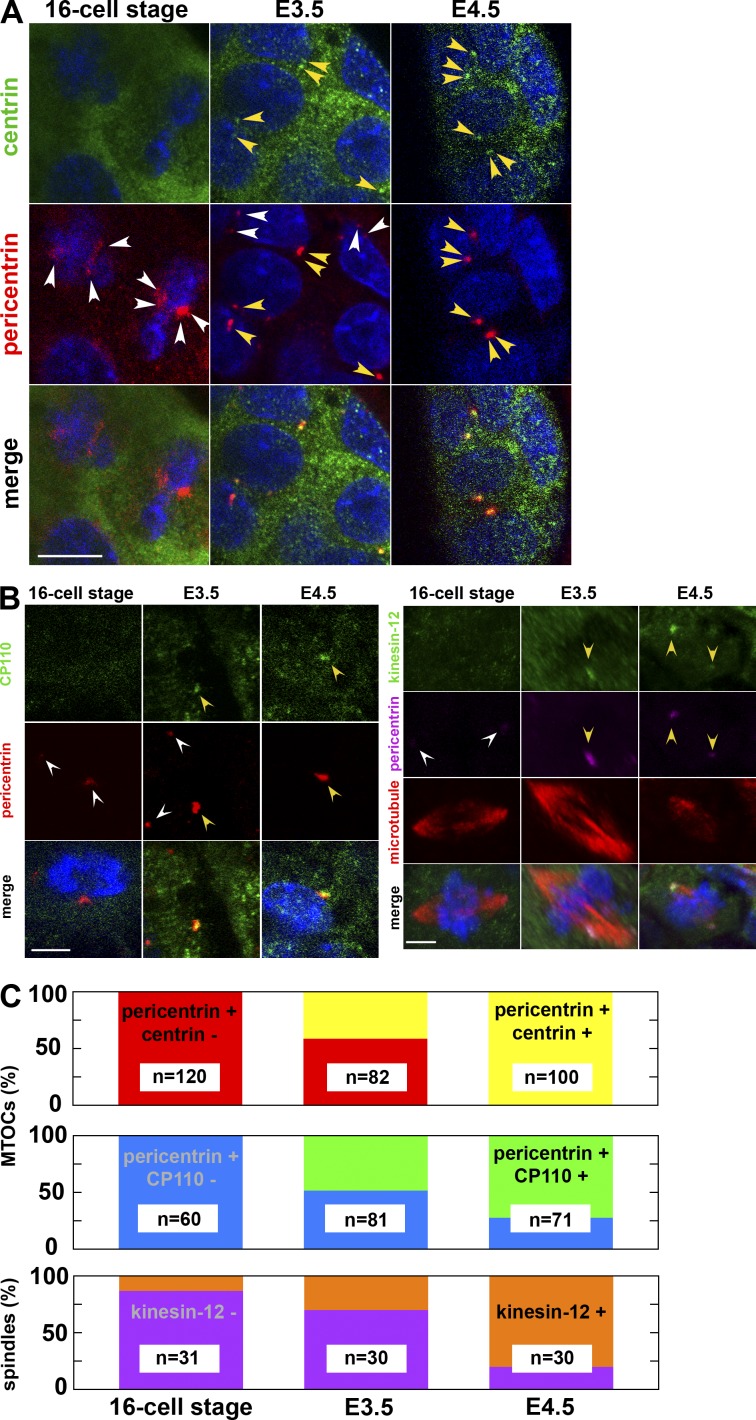
Emergence of the centrin- and CP110-positive centrosome and the kinesin-12–positive spindle in the E3.5 blastocyst. (A) Immunostaining of embryos fixed at the 16-cell stage, E3.5 (between 32- and 64-cell stages), and E4.5 (with >128 cells) and stained for DNA (blue), centrin, and pericentrin. At the 16-cell stage, all pericentrin-positive MTOCs are negative for centrin. At E3.5, some MTOCs are positive for pericentrin and negative for centrin (white arrowheads), whereas others are positive for both (yellow arrowheads). At E4.5, one or two dots positive both for pericentrin and centrin are visible in each cell (yellow arrowheads). Note that the laser intensity for centrin detection was enhanced in 16-cell stage and E3.5 embryos, resulting in the enhanced signal in the cytoplasm. Because single-section images of confocal microscopy are shown, the centrin and pericentrin signal of other cells are out of focus. Bar, 10 µm. (B, left) Immunostaining of mouse embryos fixed at the 16-cell stage, E3.5, and E4.5 and stained for DNA (blue), CP110, and pericentrin. At the 16-cell stage, MTOCs are negative for CP110. At E3.5, only some MTOCs are positive for CP110 (yellow arrowheads; white arrowheads mark those negative for CP110), whereas at E4.5, all MTOCs are positive. (right) Immunostaining of mouse embryos fixed at the 16-cell stage, E3.5, and E4.5 and stained for DNA (blue), kinesin-12, pericentrin, and microtubules. At the 16-cell stage, the spindle is negative for kinesin-12 (white arrowheads). At E3.5, only some spindles are positive for kinesin-12 (yellow arrowheads), whereas at E4.5, most of the spindles are positive (yellow arrowheads). Z-projected sections of confocal images. Bars, 5 µm. (C) The fraction of centrin-positive (yellow) and -negative (red) MTOCs, CP110-positive (green) and -negative (blue) MTOCs, and of kinesin-12–positive (orange) and –negative (violet) spindles at the 16-cell, E3.5, and E4.5 stages.