Figure 2.
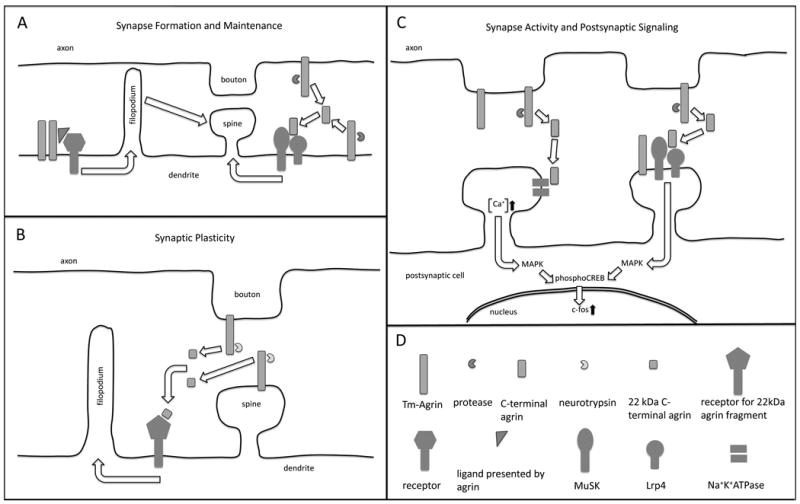
A simplified and somewhat speculative schematic of the ways in which agrin may influence synapse formation, maintenance, plasticity, activity and postsynaptic signaling, as suggested by the published findings reviewed in this article. (A left) Tm-agrin promotes the formation of dendritic filopodia by acting as a transmembrane receptor itself or by facilitation of ligand binding to an undetermined transmembrane receptor. Filopodia in turn promote the formation of spine synapses. (A right) Tm-agrin or a C-terminal proteolytic fragment activates MuSK through Lrp4 binding to promote synapse formation and/or maintenance. (B) Under conditions that promote LTP, secreted neurotrypsin generates a 22 kDa agrin C-terminal fragment. This fragment induces filopodia formation through an undetermined receptor, thus promoting new spine synapse formation. (C-left) Agrin or a C-terminal proteolytic fragment binds to the α3NKA, inhibiting the activity of Na+K+ ATPase and thus increasing excitatory synaptic activity, intracellular [Ca+] and downstream signal transduction through the MAP kinase pathway. (C-right) Tm-agrin or a C-terminal fragment activates MuSK through Lrp4 binding to elicit downstream signal transduction involved in synaptic plasticity. (D) Key to representations of the molecules mentioned in this legend. Note that Tm-agrin is actually large enough to span the synaptic cleft and act across it.
