Figure 9.
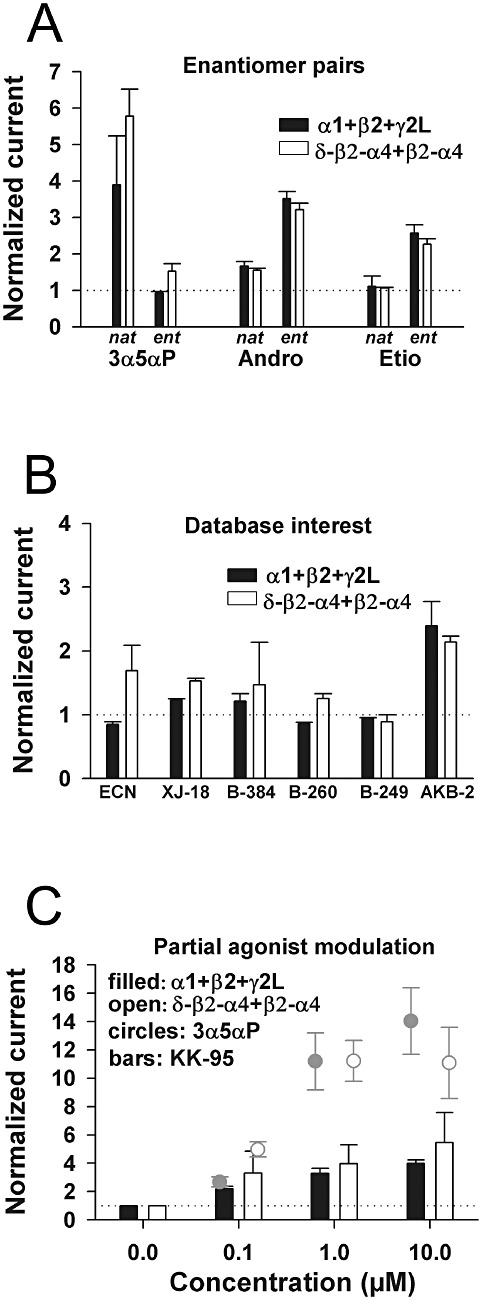
SARs for positively modulating steroid analogues are indistinguishable at extrasynaptic-like and synaptic-like subunit combinations. (A) Potentiation by enantiomer pairs (natural (nat) and unnatural (ent); 1 µM) using 0.3 µM GABA as the agonist at concatemeric α4/δ-containing receptors and an equiactive GABA concentration (2 µM) at α1 +β2 +γ2L receptors.. (B) We examined our database of structural analogues for modulators with no detectable action at α1 +β2 +γ2L receptors but with biological activity in a tadpole assay of anaesthesia. AKB-2 is a fluorescent neurosteroid analogue with weak activity at α1 +β2 +γ2L receptors in the absence of light and serves as a positive control for detection of weak effects. (C) KK-95 was tested as an unusual partial agonist analogue, with potent actions (easily detectable potentiation at 0.1 µM, similar to 3α5αP), but with weak maximum potentiation. The data for 3α5αP at the indicated concentrations are repeated from Figure 7 for reference. In all cases, analogue actions at concatemeric receptors were similar to those on α1 +β2 +γ2L synaptic-like receptors. For all experiments in panels A–C, n= 3–11 oocytes.
