Figure 6.
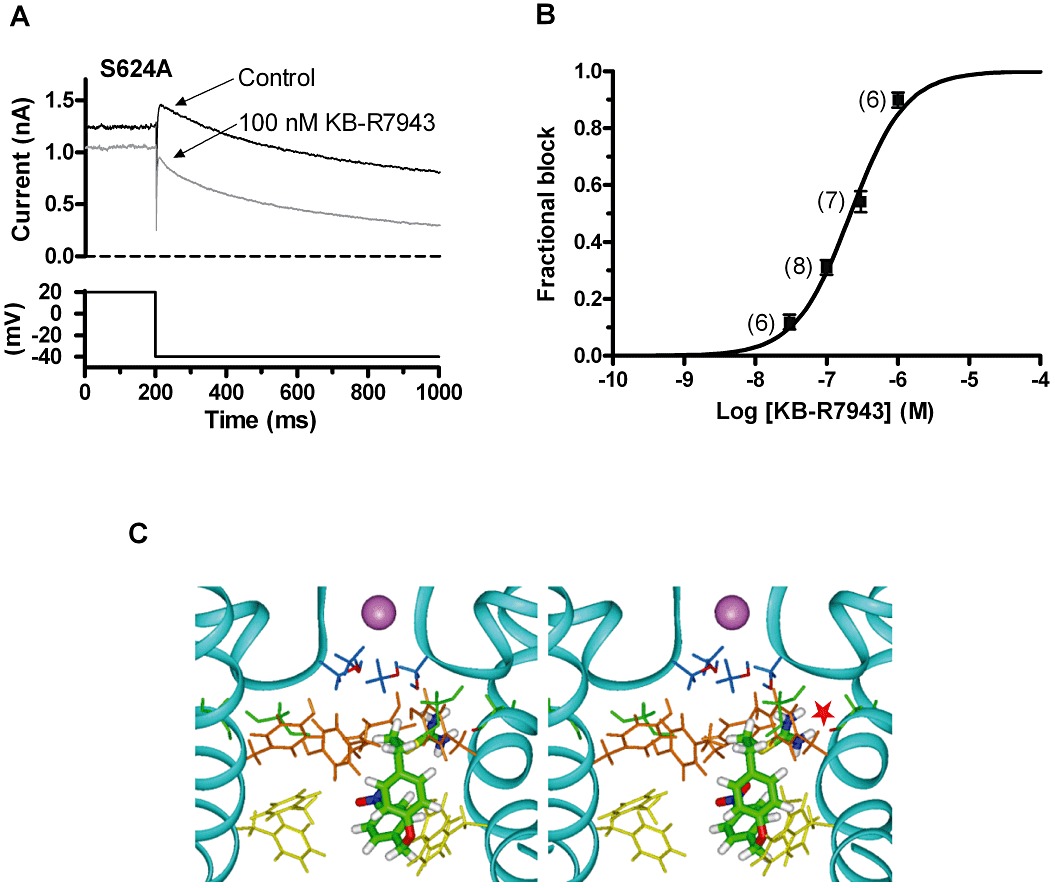
Effects of the S624A mutant on the action of KB-R7943 and simulated docking to the open hERG channel. (A) Representative records of S624A IhERG tails (upper traces) elicited on repolarization to −40 mV following a 2 s depolarization to +20 mV from −80 mV (lower trace shows corresponding portion of the voltage protocol) in control solution (standard Tyrode's solution) and following exposure to 100 nM KB-R7943. (B) Mean ± SEM fractional inhibition by SN-6 concentrations between 30 nM and 1 µM, fitted by Equation 1 to give the IC50 and nH values presented in the Results text. Numbers in parentheses indicate numbers of replicates at each drug concentration. (C) Stereoview of representative low energy score configuration for KB-R7943 docked into the open hERG channel pore homology model based on the MthK crystal structure. The protein backbone for two of the four subunits of the channel pore tetramer is indicated with a ribbon and a K+ ion (shown in mauve) occupies the S3 site in the selectivity filter. Gly648 (green) and the side chains of S624 (blue), Y652 (orange) and F656 (yellow) are shown as sticks. The red star marks a potential hydrogen bond between the thiourea group of KB-R7943 and the backbone carbonyl of G648 (green with red carbonyl oxygen).
