Abstract
Rationale
Home oxygen is the most expensive equipment item that Medicare purchases ($1.7 billion/year).
Objectives
To assess geographic differences in supplemental oxygen use.
Methods
Retrospective cohort analysis of oxygen claims for a 20% random sample of Medicare patients hospitalized for obstructive lung disease in 1999 and alive at the end of 2000.
Measurements and Main Results
While 33.7% of the 34,916 hospitalized patients used supplemental oxygen, there was more than a 4-fold difference between states and a greater than 6-fold difference between hospital referral regions with high/low utilization. Rocky Mountain States and Alaska had the highest utilization, while the District of Columbia and Louisiana had the lowest utilization. After adjusting for patient characteristics and elevation, high-utilization communities included low-lying areas in California, Florida, Michigan, Missouri, and Washington. Patients who were younger, male, white, and who had more comorbidities, more hospital admissions, and lived at higher altitudes and in areas of greater income also had higher odds of using supplemental oxygen. Residing in rural areas was associated with higher unadjusted oxygen use rates. After adjustment, patients living in large rural areas had higher odds of using oxygen than patients living in urban areas or in small rural areas.
Conclusions
There is significant geographic variation in supplemental oxygen use, even after controlling for patient and contextual factors. The Centers for Medicare & Medicaid Services should examine these issues further and enact changes that ensure patient health and fiscal responsibility.
Keywords: durable medical equipment, health services accessibility, oxygen inhalation therapy, pulmonary disease (chronic obstructive), rural health services
Supplemental oxygen significantly improves survival and quality of life in patients with obstructive lung disease.1 Its use in the home has been of increased interest as it is the most expensive equipment that Medicare purchases, costing the program $1.7 billion each year.2 A 1997 study by the Government Accounting Office found that the Centers for Medicare & Medicaid Services’ (CMS) payment rates for home oxygen were significantly more than those of the Department of Veterans Affairs.3 As a result, the Government Accounting Office recommended that CMS monitor trends in beneficiaries’ use of and access to home oxygen systems.4,5 Subsequently, CMS has taken several steps to reduce expenditures for home oxygen, including the Medicare Modernization Act of 2003, which mandated reductions in Medicare’s payments.6
However, lost in the desire to reduce oxygen payments is the potential for differences in beneficiary access based on geography. Those living at higher elevations may require supplemental oxygen earlier in the course of their lung disease because of the decreased amount of oxygen in the atmosphere. In addition, it is well established that patients in rural areas may utilize less medical care than those living in urban areas.7,8 Differences in access in rural areas may depend upon a number of variables, including patient-specific factors such as age, race, ethnicity, and perceptions of quality, as well as extrinsic factors, such as insurance coverage and health care costs.9-12 However, very little is known about the access of rural residents to durable medical equipment (oxygen, wheelchairs, and other medical supplies used at home). Some reports suggest that their access may be worse than urban residents’ access.13,14
As part of an effort to manage home oxygen use on the part of CMS, and a more general desire to understand health care utilization behaviors of patients with obstructive lung disease, we used data from the Medicare inpatient hospitalization and durable medical equipment files to assess geographic differences in the use of supplemental oxygen. In addition, we explored the relationships between patient sociodemographic, clinical, and environmental characteristics on oxygen use. This study is important because the identification of high or low users of oxygen is a first step in a quality improvement process that may reveal communities or populations that require further investigation and intervention.
Methods
We performed a retrospective cohort analysis of Medicare patients who were continuously enrolled in Parts A and B (fee-for-service) Medicare throughout the study period and were hospitalized for obstructive lung disease between January 1, 1999, and December 31, 1999. We excluded those not alive at the end of 2000 as well as those in Medicare Health Maintenance Organizations, as oxygen utilization data for these patients were unavailable. Using a 20% random sample of the Medicare Provider Analysis and Review inpatient file, we identified patients admitted to acute care hospitals with the primary diagnosis of obstructive lung disease or emphysema during 1999. The Medicare Provider Analysis and Review file contains data from all finalized claims for services provided to beneficiaries admitted to Medicare-certified inpatient hospitals. From this file, we selected those individuals whose primary diagnosis fell into the following categories: International Classification of Diseases 9th Revision codes 490.0-492.8 and 494.0-496.0. International Classification of Diseases 9th Revision codes 493.0-493.9 (asthma) were excluded. We chose to define our cohort using inpatients so that we could select individuals with severe disease who were likely candidates for supplemental oxygen. We then searched the Medicare Durable Medical Equipment records for information regarding the subsequent use of supplemental oxygen by these individuals any time after their hospitalization through the end of 2000. This durable medical equipment file contained information regarding patient age, gender, race, and home ZIP code.
We used the home ZIP code of the patient to define the rural/urban status of the beneficiary. Rural status was determined by linking this ZIP code to its Rural-Urban Commuting Area Code (RUCA).15,16 This rural-urban taxonomy was selected as RUCAs are used in a wide range of research programs and payment systems. RUCAs (Version 1.11) describe more refined geographic units than county-based systems such as the metropolitan, nonmetropolitan taxonomy and include a measure of commuting relationships. RUCAs differentiate areas based on their city/town size and work commuting patterns to larger cities and towns. The 30 RUCA designations were aggregated into 4 categories: Urban (RUCA 1.0, 1.1, 2.0, 2.1, 2.2, 3.0, 4.1, 5.1, 7.1, 8.1, 10.1), Large = Rural City (in or associated with a large rural city of 10,000 to 49,999 population, RUCA 4.0, 5.0, 6.0), Small Rural Town (in or associated with= a rural town of 2,500 to 9,999, RUCA = 7.0, 7.2, 7.3, 7.4, 8.0, 8.2, 8.3, 8.4, 9.0, 9.1, 9.2), and Isolated Rural Town (in or associated with a rural town of fewer than 2,500, RUCA 10.0, 10.2, 10.3, 10.4, 10.5). Noncity/town areas were = aggregated with the city/town where they had a strong commuting relationship.
In addition, we linked the patient’s home ZIP code to several other databases to estimate 4 other variables: median household income, elevation above sea level, state, and hospital referral region (HRR).17 Elevation above sea level was determined by linking ZIP codes to commercially available data.18 When elevation was missing from this source, first the 2003 Area Resource File (used for 2.5% of patients) and then the Web-based United States Geological Survey National Map (used for 0.1% of patients) were used to obtain elevation.19,20 The patient’s HRR was 1 of 305 distinct medical care referral regions across the United States defined by the Dartmouth Atlas of Health Care.17 Finally, we controlled for patient severity of illness by determining the number of admissions the patient had while in the cohort as well as the length of stay in the hospital. In order to control for the influence of comorbid conditions, we applied a modification of Charlson’s comorbidity index to each patient.21
Data Analysis
We first described patient sociodemographic characteristics (eg, age, race), clinical characteristics (eg, Charlson comorbidity index), environmental characteristics (eg, elevation), and oxygen use by the 4 RUCA types. Standard statistical tests were employed including overall chisquare tests and analysis of variance. We then calculated oxygen use rates in both states and HRRs, identifying states and HRRs with unadjusted rates of oxygen use that were more or less than 2 SDs from the corresponding mean rates of use. We also created a map displaying the distribution of states’ unadjusted oxygen supplementation rates using logical breakpoints. Next, we determined which states and HRRs had high or low utilization of supplemental oxygen after adjustment for patient sociodemographic, clinical, and environmental variables. In these multivariate patient-level logistic regression analyses, the dependent variable was occurrence or not of any patient oxygen claim. We report those states and HRRs where the odds ratios were greater than 2 SDs above or below the overall mean odds ratio. Last, we conducted a multivariate patient-level logistic regression analysis to identify the association between oxygen use and residence in different RUCA types.
Results
We identified 35,588 Medicare patients with a hospitalization in 1999 who met our study criteria. We excluded 672 (1.9%) patients who were missing at least 1 geographic identifier (598 without an HRR code and 74 without a RUCA code), leaving 34,916 patients in our final cohort. Patient characteristics by rural/urban categories are displayed in Table 1. Of the study patients, 11,766 (33.7%) had a claim for home oxygen between hospitaldischarge and December 31, 2000. Patients living in rural areas had higher rates of home oxygen use than those in urban areas; however, the elevations of these rural areas, on average, were nearly twice that of the urban areas. Those living in rural areas were the most likely to be white or male and have the shortest lengths of stay and the lowest income. The rural-urban differences for the subset of the cohort that had an oxygen claim were negligible, except sex. Within this subset, those living in urban areas were most likely to be male. Finally, the RUCA groups were very similar in terms of number of admissions and Charlson comorbidity scores.
Table 1.
Patient Characteristics by Rural and Urban Residence Location†
| Urban | Large Rural | Small Rural | Isolated Small Rural | Total | |
|---|---|---|---|---|---|
| All beneficiaries hospitalized with obstructive lung disease | |||||
| Number of patients | 22,586 | 4,589 | 4,319 | 3,422 | 34,916 |
| % with oxygen claim*** | 32.6% | 36.8% | 34.3% | 35.9% | 33.7% |
| Mean number of oxygen claims (SD)*** | 8.36 (16.8) | 9.65 (17.3) | 9.42 (17.7) | 9.91 (17.7) | 8.81 (17.1) |
| Mean age (SD)*** | 75.19 (10.9) | 74.30 (10.6) | 74.52 (10.4) | 74.88 (10.6) | 74.96 (10.8) |
| %male*** | 43.6% | 47.9% | 47.7% | 51.8% | 45.5% |
| % nonwhite*** | 14.3% | 8.1% | 7.3% | 5.8% | 11.8% |
| Mean number of admissions (SD)** | 1.37 (0.9) | 1.35 (0.8) | 1.42 (1.0) | 1.40 (1.0) | 1.38 (0.9) |
| Mean hospital length of stay in days (SD)*** | 7.15 (7.9) | 6.13 (5.7) | 5.68 (4.8) | 5.53 (4.9) | 6.67 (7.1) |
| Mean Charlson comorbidity index (SD)** | 1.06 (1.3) | 1.04 (1.3) | 0.99 (1.3) | 1.00 (1.3) | 1.04 (1.3) |
| Mean county elevation in feet (SD)*** | 581 (855) | 988 (1,116) | 938 (1,062) | 1,069 (1,061) | 726 (962) |
| Mean ZIP code-based median household income (SD)*** |
$40,970 ($16,274) | $30,446 ($6,827) | $27,175 ($6,102) | $25,824 ($6,335) | $36,396 ($15,021) |
| Beneficiaries hospitalized with obstructive lung disease and had an oxygen claim | |||||
| Number of patients | 7,364 | 1,690 | 1,483 | 1,229 | 11,766 |
| Mean number of oxygen claims (SD)*** | 25.64 (20.5) | 26.21 (19.4) | 27.43 (20.4) | 27.60 (19.7) | 26.15 (20.3) |
| Mean age (SD)** | 74.11 (9.7) | 73.29 (9.3) | 73.65 (9.3) | 74.00 (9.3) | 73.92 (9.6) |
| %male*** | 50.1% | 56.2% | 57.5% | 58.6% | 52.8% |
| % nonwhite*** | 11.2% | 6.5% | 5.3% | 4.5% | 9.0% |
| Mean number of admissions (SD)** | 1.60 (1.2) | 1.54 (1.0) | 1.69 (1.3) | 1.63 (1.3) | 1.61 (1.2) |
| Mean hospital length of stay in days (SD)*** | 7.25 (6.7) | 6.31 (5.8) | 5.83 (4.5) | 5.90 (4.8) | 6.80 (6.2) |
| Mean Charlson comorbidity index (SD) | 1.14 (1.4) | 1.10 (1.4) | 1.08 (1.3) | 1.10 (1.4) | 1.13 (1.4) |
| Mean county elevation in feet (SD)*** | 693 (1,035) | 1,129 (1,280) | 1,031 (1,180) | 1,200 (1,264) | 851 (1,136) |
| Mean ZIP code-based median household income (SD)*** |
$40,917 ($15,544) | $30,941 ($6,800) | $27,395 ($6,131) | $26,489 ($6,517) | $36,272 ($14,301) |
Asterisks indicate statistically significant differences across the 4 geographic locations:
P ≤ .05
P ≤ .01
P ≤ .001.
Aggregations of Rural-Urban Commuting Areas.
Missing values: All beneficiaries: race: urban 100, large rural 20, small rural 18, isolated small rural 16; length of stay: urban 172, large rural 30, small rural 29, isolated small rural 23; ZIP code-based median household income: urban 1, large rural 0, small rural 0, isolated small rural 0. Beneficiaries who had an oxygen claim: race: urban 24, large rural 6, small rural 6, isolated small rural 5; length of stay: urban 43, large rural 10, small rural 6, isolated small rural 9.
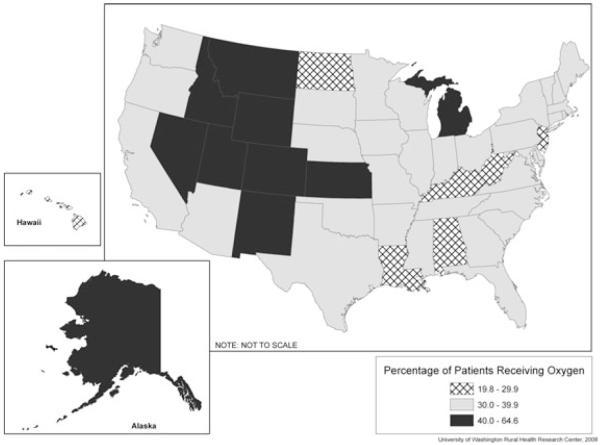
Figure 1 displays a state-level analysis. In general, the higher utilization areas were in the mountain states, while low-utilization areas were in the eastern and southern regions. There was more than a 4-fold difference between the highest and lowest utilization states. These data can be found in Supplementary Table 1 (available online only), which shows the percent of study patients who used supplemental oxygen by state.
Figure 1.
Unadjusted Percentage of Study Patients Receiving Oxygen Supplementation.
There were several HRRs that were greater than 2 SDs above or below the HRR mean unadjusted utilization rates for supplemental oxygen. High-utilization HRRs included Colorado Springs, Fort Collins, Greeley, and Pueblo, Colorado; Idaho Falls, Idaho; Traverse City, Michigan; Amarillo, Texas; Salt Lake City and Ogden, Utah; and Casper, Wyoming. High-utilization states (2 SDs above the state mean unadjusted utilization rate) included Colorado, Utah, and Wyoming (data not shown). Only the District of Columbia was classified as a low-utilization state (2 SDs below the mean unadjusted utilization rate), while 4 HRRs—Lafayette, Louisiana; New Brunswick, New Jersey; Grand Forks, North Dakota; and Harlingen, Texas—fell into the low-utilization-rate category. These data may be found in Table 2.
Table 2.
HRRs 2 SDs Above and Below the Unadjusted Mean Oxygen Utilization Rate and the Adjusted Mean Oxygen Utilization Odds Ratio
| Unadjusted % Using Oxygen | N | |
|---|---|---|
| HRR unadjusted oxygen utilization rate >2 SDs above the mean HRR | ||
| oxygen utilization rate | ||
| Ogden, UT | 76.0 | 25 |
| Pueblo, CO | 76.0 | 25 |
| Idaho Falls, ID | 71.4 | 21 |
| Casper, WY | 63.9 | 36 |
| Fort Collins, CO | 62.5 | 32 |
| Greeley, CO | 62.5 | 32 |
| Colorado Springs, CO | 62.1 | 66 |
| Amarillo, TX | 60.7 | 84 |
| Traverse City, MI | 60.7 | 28 |
| Salt Lake City, UT | 59.7 | 119 |
| HRR unadjusted oxygen utilization rate >2 SDs below the mean HRR | ||
| oxygen utilization rate | ||
| Harlingen, TX | 14.8 | 54 |
| New Brunswick, NJ | 13.7 | 102 |
| Lafayette, LA | 12.0 | 92 |
| Grand Forks, ND | 11.4 | 35 |
| HRR adjusted* odds ratio for oxygen utilization >2 SDs above the mean HRR odds ratio | ||
| Ogden, UT | 76.0 | 25 |
| Pueblo, CO | 76.0 | 25 |
| Idaho Falls, ID | 71.4 | 21 |
| Amarillo, TX | 60.7 | 84 |
| Traverse City, MI | 60.7 | 28 |
| Lakeland, FL | 53.2 | 62 |
| La Crosse, WI | 53.1 | 32 |
| Saginaw, MI | 51.9 | 104 |
| Flint, MI | 50.0 | 88 |
| Grand Rapids, MI | 50.0 | 114 |
| Olympia, WA | 50.0 | 30 |
| South Bend, IN | 47.5 | 99 |
| Redding, CA | 46.9 | 49 |
| Cape Girardeau, MO | 46.7 | 45 |
| HRR adjusted* odds ratio for oxygen utilization >2 SDs below the mean HRR odds ratio | ||
| None | ||
Multiple logistic regression analyses adjusted for age, sex, race, number of admissions, Charlson comorbidity index, elevation, and ZIP code-based median household income.
After adjusting for patient and contextual characteristics (including elevation above sea level), a somewhat different set of states and HRRs were identified as high oxygen utilizers (2 SDs above the mean odds ratio). These included the state of Alaska, as well as the HRRs of Redding, California; Pueblo, Colorado; Lakeland, Florida; Idaho Falls, Idaho; South Bend, Indiana; Traverse City, Saginaw, Flint, and Grand Rapids, Michigan; Cape Girardeau, Missouri; Amarillo, Texas; Ogden, Utah; Olympia, Washington; and La Crosse, Wisconsin. After this same adjustment, low oxygen utilization areas (2 SDs below the mean odds ratio) included the District of Columbia and Louisiana (data not shown), but no HRRs. These data may also be found in Table 2.
In a multivariate logistic regression analysis (Table 3) that examined various sociodemographic, clinical, and environmental characteristics and the use of oxygen, patients living in large rural areas were significantly more likely to use oxygen than patients living in urban areas (P = .016). Within all rural areas, patients living in large rural areas were significantly more likely than those in small rural areas (P .030), but not significantly more likely than those in=isolated small rural areas (P = .748), to use oxygen. In addition, patients who were younger, male, white, had a higher Charlson comorbidity index, a greater number of hospital admissions, and lived at higher altitudes and in areas of greater income had higher odds of using supplemental oxygen than their counterparts.
Table 3.
Odds of Supplemental Oxygen Use by Patient Residence Location, Sociodemographic, Clinical, and Environmental Characteristics (n = 34,761)
| Patient Characteristics | Odds Ratio (CI*) | P Value |
|---|---|---|
| Residence location | ||
| Urban | 0.91 (0.85, 0.98) | .016 |
| Large rural | 1.0 (ref) | — |
| Small rural | 0.90 (0.83, 0.99) | .031 |
| Isolated small rural | 0.98 (0.89, 1.09) | .754 |
| Age (years) | ||
| <65 | 1.46(1.35, 1.58) | .000 |
| 65-69 | 1.64 (1.52, 1.76) | .000 |
| 70-74 | 1.67 (1.56, 1.78) | .000 |
| 75-79 | 1.49 (1.40, 1.59) | .000 |
| 80+ | 1.0 (ref) | — |
| Sex | ||
| Male | 1.53 (1.46, 1.61) | .000 |
| Female | 1.0 (ref) | — |
| Race | ||
| White | 1.57 (1.46, 1.69) | .000 |
| Nonwhite | 1.0 (ref) | — |
| Number of hospital admissions during study period | ||
| 1 | 1.0 (ref) | — |
| 2 | 2.02 (1.90, 2.16) | .000 |
| 3+ | 3.26 (3.00, 3.53) | .000 |
| Elevation of residence location | ||
| 0-500 feet | 1.0 (ref) | — |
| 501-1,000 feet | 1.09 (1.03, 1.15) | .002 |
| 1,001-2,000 feet | 1.17 (1.08, 1.26) | .000 |
| 2,001-3,000 feet | 1.25 (1.08, 1.44) | .003 |
| 3,001-4,000 feet | 1.89 (1.57, 2.27) | .000 |
| 4,001 + feet | 2.85 (2.48,3.28) | .000 |
| Median household income in ZIP code | ||
| ≤$20,000 | 1.0 (ref) | — |
| $20,001-$25,000 | 1.20 (1.07, 1.35) | .002 |
| $25,001-$30,000 | 1.27 (1.13, 1.42) | .000 |
| $30,001-$40,000 | 1.37 (1.22, 1.53) | .000 |
| $40,001-$50,000 | 1.41 (1.26, 1.59) | .000 |
| $50,001 + | 1.27 (1.12, 1.43) | .000 |
| Charlson comorbidity index | ||
| 0 | 1.0 (ref) | — |
| 1 | 1.11 (1.05, 1.17) | .000 |
| 2 | 1.05(0.98, 1.13) | .140 |
| 3+ | 1.08(1.00, 1.17) | .056 |
CI, confidence interval.
Missing values: race/ethnicity 154, income 1.
Discussion
Our study reveals that over one third of all Medicare patients admitted for obstructive lung disease utilized supplemental oxygen within 1 to 2 years of hospital discharge. However, there is significant statewide variation in the use of supplemental oxygen after hospitalization, with the highest use occurring in the Rocky Mountain states. Analysis of HRRs suggests that after controlling for sociodemographic, clinical, and environmental factors, some low-lying communities such as Lakeland, Florida, and Olympia, Washington, have very high utilization rates.
The unadjusted results suggest few rural/urban differences in the rates of supplemental oxygen use. However, our controlled analysis suggests that supplemental oxygen use was higher in large rural areas compared to urban areas and small rural areas. Previous studies have shown that the medical care received in large rural areas is similar in quality to that of urban areas. For example, Rosenblatt et al found that patients living in large remote rural areas received the highest quality diabetes care,22 while Stearns et al found that those in large rural areas reported the highest rates of satisfaction with care anywhere.23 Additional research has demonstrated that patients receiving care from hospitals in large rural areas generally had guideline adherence rates for acute myocardial infarction close to that of urban areas.8 There are several explanations for such findings. Many of the large rural towns/cities are vital economic entities with growing populations, and their hospitals are referral sites for the surrounding areas. These rural towns/cities of 10,000 to 49,000 often have an adequate supply of both primary care and specialty care physicians along with the associated medical infrastructure.
There are several limitations to our findings. First, we defined our cohort through an initial hospitalization. Our findings may not generalize to those individuals who started supplemental oxygen as an outpatient. In addition, we had limited data on patient severity of illness. Because we were dealing with administrative data, we had no specific measures of pulmonary function. Thus, we were forced to infer patient severity of illness by controlling for the number of hospital admissions and patient length of stay. Another limitation is the small sample size in many of the HRRs, resulting in oxygen supplementation rates with very large confidence intervals. For this reason, we present results only for those HRRs above or below 2 SDs from the mean oxygen supplementation rates. We do not have any data on patient outcomes. Although we have documented that significant variation in supplemental oxygen utilization exists between geographic areas, it is unclear what the appropriate rate of supplemental oxygen utilization is after hospitalization for obstructive lung disease.14,24
To add to the robustness of our modeling, we performed additional sensitivity analyses, which included a measure of air pollution as well as state as a fixed effect in our multivariate regression analysis. Using a methodology described previously, we identified patient exposure to particulate matter less than 2.5 μm on aerodynamic diameter, based on ZIP code.25 Particulate matter less than 2.5 μm was chosen as it is an air pollutant that has been linked to mortality.25 Particulate matter less than 2.5 μm data were collected by the Environmental Protection Agency and were available for the year 2000. Patients were included if they were within 100 miles of an air pollution monitor, which required us to drop 569 (1.6%) subjects. Our main findings did not change in these secondary regression analyses, and thus only the original results are displayed.
Despite these limitations, this study has important implications. To our knowledge, this is the first time geographic differences in the utilization of supplemental oxygen have been examined in detail. While we failed to find dramatic rural/urban differences in oxygen utilization, the reasons for this need to be investigated further. If indeed supplemental oxygen is delivered in a manner that is less related to rural location, it may be a model for other types of service delivery where rural location is an issue. In addition, we have identified significant variations between states and HRRs. Given that there is an over 4-fold difference between the high- and low-utilization states and an over 6-fold difference between high- and low-utilization HRRs, that urban and small rural areas have the lowest adjusted oxygen use rates, and that CMS pays nearly $2 billion per year for these services, further examination of why these variations exist is warranted. This investigation should include an assessment of practice guidelines, as well as ways to reduce administrative complexity and fraud. CMS, through its Quality Improvement Organizations and Durable Medical Equipment Regional Carriers, has the means to examine these issues in detail and enact changes that will improve both patient health and fiscal responsibility.
Supplementary Material
Acknowledgments
This study was supported through the WWAMI Rural Health Research Center with funding from the federal Office of Rural Health Policy, Health Resources and Services Administration, Public Health Service (Grant No. 5U1CRH00035-02). Additional resources were provided by the Centers for Medicare and Medicaid Services and the Clinical Research Center, National Institutes of Health. The authors would like to thank Pam Green, PhD, for calculating the comorbidity index used in this analysis as well as Dr. Joel Kaufman who provided data and assisted in estimates regarding the effect of air pollution.
Footnotes
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1 Percent of Patients With an Oxygen Claim by State
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Crockett AJ, Moss JR, Cranston JM, Alpers JH. Domiciliary oxygen for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2000;(2):CD001744. doi: 10.1002/14651858.CD001744. [DOI] [PubMed] [Google Scholar]
- 2.Dunne PJ. The demographics and economics of long-term oxygen therapy. Respir Care. 2000;45(2):223–228. discussion 228-230. [PubMed] [Google Scholar]
- 3.Reis W, Putallaz F, Rubins S. Medicare: Comparison of Medicare and VA Payment Rates for Home Oxygen. Government Accounting Office; Washington, DC: 1997. GAOMEHS-97-120R. [Google Scholar]
- 4.Kelly A, Putallaz F, Rubins S. Access to Home Oxygen Largely Unchanged; Closer HCFA Monitoring Needed. General Accounting Office; Washington, DC: 1999. GAO/HEHS-99-56. [Google Scholar]
- 5.Putallaz F, Rubins S. Home Oxygen Program Warrants Continued HCFA Attention. Government Accounting Office; Washington, DC: 1997. GAO/HEHS-98-17. [Google Scholar]
- 6.Office of the Inspector General . Medicare and FEHB Payment Rates for Home Oxygen Equipment. Department of Health and Human Services; Washington, DC: [Accessed December 21, 2009]. Mar, 2005. OEI-09-03-00160. Available at: http://oig.hhs.gov/oei/reports/oei-09-03-00160.pdf. [Google Scholar]
- 7.Grossman DC, Krieger JW, Sugarman JR, Forquera RA. Health status of urban American Indians and Alaska Natives. A population-based study. JAMA. 1994;271(11):845–850. [PubMed] [Google Scholar]
- 8.Baldwin LM, MacLehose RF, Hart LG, Beaver SK, Every N, Chan L. Quality of care for acute myocardial infarction in rural and urban US hospitals. J Rural Health. 2004;20(2):99–108. doi: 10.1111/j.1748-0361.2004.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 9.Borders TF, Rohrer JE. Rural residence and migration for specialty physician care. Health Care Manage Rev. 2001;26(3):40–49. doi: 10.1097/00004010-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Borders TF, Rohrer JE, Hilsenrath PE, Ward MM. Why rural residents migrate for family physician care. J Rural Health. 2000;16(4):337–348. doi: 10.1111/j.1748-0361.2000.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 11.Zhang P, Tao G, Anderson LA. Differences in access to health care services among adults in rural America by rural classification categories and age. Aust J Rural Health. 2003;11(2):64–72. doi: 10.1046/j.1440-1584.2003.00454.x. [DOI] [PubMed] [Google Scholar]
- 12.Casey MM, Thiede Call K, Klingner JM. Are rural residents less likely to obtain recommended preventive healthcare services? Am J Prev Med. 2001;21(3):182–188. doi: 10.1016/s0749-3797(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 13.Research and Training Center on Disability in Rural Communities . Rural Disability and Rehabilitation Research Progress Report #4. University of Montana; Missoula, MT: [Accessed October 16, 2008]. Rural managed care and disability: emerging issues from preliminary interviews and case studies. Available at: http://rtc.ruralinstitute.umt.edu/health/mancareprgrpt.htm. [Google Scholar]
- 14.Ringbaek TJ, Lange P, Viskum K. Geographic variation in long-term oxygen therapy in Denmark: factors related to adherence to guidelines for long-term oxygen therapy. Chest. 2001;119(6):1711–1716. doi: 10.1378/chest.119.6.1711. [DOI] [PubMed] [Google Scholar]
- 15.Morrill R, Cromartie J, Hart LG. Metropolitan, urban, and rural commuting area: toward a better depiction of the U.S. settlement system. Urban Geogr. 1999;20:727–748. [Google Scholar]
- 16.WWAMI Rural Health Research Center [Accessed February 21, 2006];Rural-Urban Commuting Area Codes (RUCAs) Available at: http://depts.washington.edu/uwruca/
- 17.Wennberg J, Cooper M, Center for the Evaluative Clinical Sciences Staff . The Dartmouth Atlas of Health Care. Center for the Evaluative Clinical Sciences, Dartmouth Medical School; Hanover, NH: 1998. [PubMed] [Google Scholar]
- 18.Hexa Software Development Center [Accessed November 1, 2005];United States ZIP code data file gold edition. Available at: http://www.hexa-soft.com.
- 19.United States Geological Survey [Accessed June 30, 2008];The national map viewer. Available at: http://nmviewogc.cr.usgs.gov/viewer.htm.
- 20.Health Resources and Services Administration . Area resource file, February 2003 release. National Center for Health Workforce Analysis, Bureau of Health Professions, Health Resources and Services Administration, Department of Health and Human Services; Fairfax, VA: 2003. [Google Scholar]
- 21.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 22.Rosenblatt RA, Baldwin LM, Chan L, et al. Improving the quality of outpatient care for older patients with diabetes: lessons from a comparison of rural and urban communities. J Fam Pract. 2001;50(8):676–680. [PubMed] [Google Scholar]
- 23.Stearns SC, Slifkin RT, Edin HM. Access to care for rural Medicare beneficiaries. J Rural Health. 2000;16(1):31–42. doi: 10.1111/j.1748-0361.2000.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 24.Chaney JC, Jones K, Grathwohl K, Olivier KN. Implementation of an oxygen therapy clinic to manage users of long-term oxygen therapy. Chest. 2002;122(5):1661–1667. doi: 10.1378/chest.122.5.1661. [DOI] [PubMed] [Google Scholar]
- 25.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.