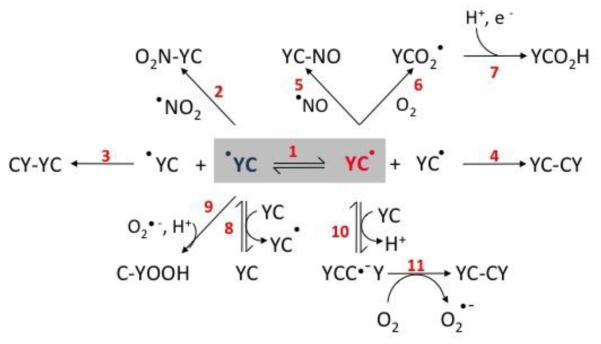
Figure 1. Electron transfer and oxidation reactions in Tyr-Cys-containing peptides.
The figure shows reactions involved in uni- and bimolecular processes related to radical-mediated oxidations in Tyr-Cys-containing peptides in the presence of oxygen, nitric oxide and nitrogen dioxide.The numbers in red indicate discrete reactions as follows. 1. Intramolecular electron transfer; 2. Combination reaction between tyrosyl radical and ●NO2 to yield 3-nitrotyrosine peptide; 3. Tyrosyl radical dimerization yielding 3,3′-di-tyrosine peptide; 4. Thiyl radical dimerization yielding disulfide peptide; 5. Combination reaction between cysteinyl radical and ●NO to yield S-nitrosocysteine peptide; 6-7. Thiyl radical reaction with oxygen to yield thiylperoxyl radical peptide (6) and subsequent rearrangement and reduction tosulfinic acid derivative (7); 8. Intermolecular electron transfer; 9. Reaction between tyrosyl radical and superoxide yielding tyrosine hydroperoxide; 10-11. Reaction of cysteinyl radical peptide with tyrosine-cysteine peptide to yield the disulfide radical anion (10), followed by the reduction of molecular oxygen to yield O2●- and the corresponding disulfide peptide (11).