Introduction
Pain management in dentistry can be a real challenge. The key to managing pain lies in understanding whether what you do to patients will or will not create an inflammatory response, which is what activates the pain-producing mediators in tissue. Generally, procedures on hard tooth structure that do not involve the pulp create little or no inflammatory response, but, when soft tissues are traumatised, a pain response can be expected (1).
Oral medications that reduce pain, administered pre or postoperatively, improve clinical outcomes, making them an integral part of dental practice (2).
Analgesic medications in dentistry are indicated for the relief of acute pain, postoperative pain, and chronic pain, and for controlling adjunctive intraoperative pain. In addition these medications can be given preoperatively, to mitigate both postoperative pain and reduce postoperative pain medication requirement (2).
Alleviating pain is of the utmost importance when treating dental patients, as it is prevalent and has far-reaching implications, for both the patient and the clinician (3). The major cause of pain is thought to be the release of inflammatory mediators that activate sensory nocioceptors surrounding the tooth (4). The resultant stimulation of both central and peripheral mechanisms (5) is referred to as hyperalgesia and defined as an increase in perceived magnitude of a painful stimulus (6). Given that the mechanisms involved are occurring at the periphery, an anti-inflammatory agent should be used to control this process. Non-steroidal anti-inflammatory drugs (NSAIDs) are among the most widely prescribed analgesics for management of post-operative pain in dental patients (7).
NSAIDs that have been approved by the US Food and Drug Administration (FDA) for OTC analgesic use can be divided into three groups: salicylates (i.e. aspirin, salycilic acid, diflunisal), proprionic acid derivatives (i.e. ibuprofen, naproxen, and ketoprofen) and the para-aminophenol derivative acetaminophen.
The analgesic effect of NSAIDs is primarily the result of their inactivation of cyclooxygenase, an enzyme that converts arachidonic acid into eicosanoids such as prostaglandins and leukotrienes (8,9,10). Two forms of cyclooxygenase have been identified: COX-1, which is constitutive and exists in the stomach, intestines, kidneys, and platelets, and COX-2, which is expressed as part of the inflammatory process (11). Ibuprofen is a nonselective inhibitor of cyclooxygenase and is available as both a prescription and over-the-counter (OTC) product (12).
Conversely, celecoxib, introduced as a prescription drug in January 1999, selectively inhibits the COX-2 form of the enzyme (13). More recently, celecoxib was approved by the FDA for acute analgesia with directions to use 400 mg as an initial loading dose followed by 200 mg every 24 hours.
Acetaminophen, a para-aminophenol derivative, posed a problem with regard to this classification, as it has analgesic and antipyretic actions but little or no anti-inflammatory activity (14); this led to the suggestion, several years ago, that there is a further COX in the brain, named COX-3(15). To date, however, the existence of this putative COX-3 has not been proven, the presence of a COX-1 variant seeming more likely, even though the presence of another COX gene has not been ruled out (16).
The ability of NSAIDs to inhibit both COX-1 and COX-2 may increase the efficacy of this class of drugs. Indeed, when the lipoxin pathway is activated in the presence of COX-1 inhibitors, acetylation of the COX-2 enzyme occurs to inhibit further production of prostanoids through arachidonic acid metabolism while inducing the synthesis of 15-R-hyroxy-(p)-eicosatetraenoic acid that is transformed to 5(6)-epoxytetraene and then into15-epi-lipoxins or into aspirin-triggered 15-epi-lipoxins (ATLs). Both 15-epi-lipoxins and ATLs control the resolution phase of acute inflammation and promote lesion healing (17,18).
The generation of lipoxins or ATLs triggered by “first-phase” proinflammatory lipid mediators may explain the potentially serious cardiovascular consequences of the chronic use of selective COX-2 antagonists (see later). Inflammation is a multifactorial process, therefore a single “paninflammatory” agent cannot antagonise all deleterious pathways involved while preserving the resolution pathways (19).
While COX-2 inhibitors may provide a therapeutic advantage for patients requiring chronic NSAID therapy who are at increased risk of developing gastrointestinal (GI) adverse events, for the routine treatment of moderate to severe acute pain in the general population, ibuprofen provides faster and better analgesic efficacy without any apparent increased safety risk. Ibuprofen has been proven to be safe and effective in the relief of postoperative dental pain in adults.
Ibuprofen pharmacology
Ibuprofen, a 2-proprionic acid derivative discovered by the research arm of the British Boots Group in the 1960s, is a peripherally acting analgesic with a potent anti-inflammatory action that works through a reversible and balanced COX-1/COX-2 inhibition (20). Previously, Seymour and Walton documented that drugs with both analgesic and anti-inflammatory action are able to control postoperative dental pain (21). Indeed, ibuprofen has been evaluated extensively in postoperative dental pain and several studies support its efficacy (22,23,24).
Ibuprofen exists as a racemic mixture of both R(−) and S( +) enantiomers, and its anti-inflammatory, analgesic and anti-platelet effects (determined by cyclooxygenase inhibition) are related to the S(+) enantiomer (25,26,27).
By contrast R(−) ibuprofen is less active as a prostaglandin (PG) synthesis inhibitor but has shown some pharmacological properties relevant to the anti-inflammatory actions of ibuprofen (28). However, 50–60% of the R(−)-form of ibuprofen is metabolically converted to the S(+) form in the intestinal tract and liver after oral absorption (29).
The pharmacokinetic profile of ibuprofen has been examined in both single-dose and multiple-dose studies in children. Following a single dose of 5 mg/kg and 10 mg/kg ibuprofen in children aged between 2 and 11 years, peak plasma concentrations of ibuprofen were achieved in under 2 hours with a half-life of less than 2 hours (30). Multiple-dose pharmacokinetics of ibuprofen (20–40 mg/kg/day ibuprofen syrup in divided doses), administered to children affected by juvenile arthritis with a mean age of 8.8 years (range 1.5–16 years), indicate that, at steady state, peak plasma levels of 100–150 mol/l are achieved within 1–2 hours after dosing with a half-life of 2 hours (31).
These data indicate that the pharmacokinetic profile of ibuprofen is not different in younger and older children and that the pharmacokinetic profile of ibuprofen in children is similar to that observed in adults.
The anti-inflammatory and analgesic properties of NSAIDs result primarily from their blocking of cyclooxygenase activity at the site of tissue injury, leading to the inhibition of PG synthesis (32) and the secondary inhibition of the sensitisation of nociceptive nerve endings (33). However, other independent effects have also been documented. Nielsen et al. (34), in a clinical trial, suggested that ibuprofen might also act centrally on PG release, or have a direct effect on peripheral nerve endings (35).
Moreover, it has been demonstrated in experimental studies that several NSAIDs, such as ibuprofen, ketorolac, and flurbiprofen, are able to inhibit the fatty acid amide hydrolase (FAAH), the enzyme that degrades anandamide (36, 37) and leads to increased anandamide levels. In addition, it has been demonstrated that the combination of ibuprofen with anandamide produced a synergistic analgesic effect in the formalin test which is mediated by CB1 and partially by CB2 cannabinoid receptors (38).
Indeed, this modulation of the endogenous cannabinoids, through blocking of FAAH, conferred a better anti-nociceptive effect than endocannabinoids given alone (39). Another mechanism of action involved the beta-endorphin that is secreted in response both to surgical stress and during postoperative pain (40). Dionne and McCullagh, in 1998, found that the administration of ibuprofen following pain onset in an oral surgery model resulted in decreased plasma beta-endorphin levels coinciding with a reduction in pain. They suggested that ibuprofen suppresses pituitary beta-endorphin release and produces analgesia, presumably by suppressing nociceptive activation of the pituitary-adrenal axis (41).
Another reported effect of NSAIDs is reduction of oedema, which is a typical sign of tissue injury-induced inflammation during the acute postoperative sequelae of dental procedures. Ibuprofen at both 1200 mg daily for three days and 2400 mg daily for two days significantly suppressed oedema formation 48 hours after oral surgery (42,43), even though better efficacy has been documented with ibuprofen 1200 mg per day plus 32 mg of methylprednisolone (44).
Absorption and distribution
Ibuprofen is rapidly absorbed from the upper gastrointestinal tract (Tmax <0.25 hours for granules and about 2 hours for tablets), even though absorption is delayed if ibuprofen is administered with food (45). The plasma S/R ratio is dependent on the time-release characteristics of the drug, higher ratios being obtained with sustained-release compared with immediate-release formulations.
Like most other NSAIDs, ibuprofen has a short half-life (2.1 hours) (Table 1), which, even should repeated administration is required, is able to reduce the development of side effects (see later).
Table 1.
Half-lives of NSAIDs in healthy patients.
| Drug | Half-life (h) |
|---|---|
| Aspirin | 0.2 |
| Diclofenac | 1.1 |
| Ketoprofen | 1.8 |
| Ibuprofen | 2.1 |
| Flurbiprofen | 3.8 |
| Ketorolac | 5.1 |
| Naproxen | 14 |
| Celecoxib | 16 |
| Piroxicam | 57 |
Ibuprofen and the other NSAIDs tend to have similarly small values for total body clearance (0.01 to 0.05 L/kg/min) and volume of distribution (10 to 15 L for an individual weighing 70 kg), and extensive binding to plasma proteins (90 to 99%; except for acetaminophen, which is approximately 20% bound) (46,47,48).
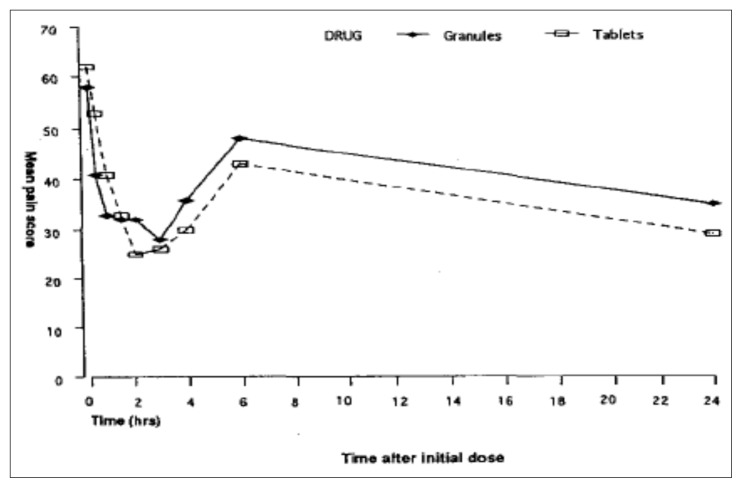
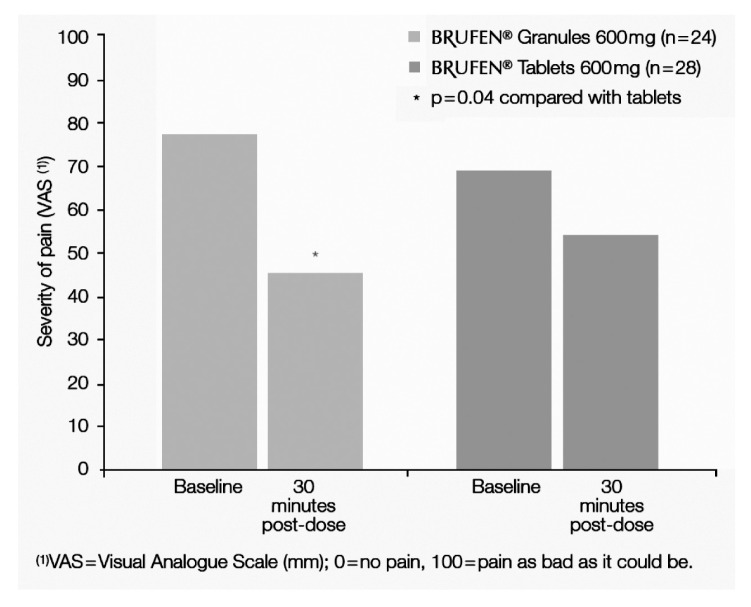
Moreover, Seymour et al. (49), evaluating patients with postoperative pain after third molar surgery, reported that soluble ibuprofen 400 mg provided an earlier onset of pain relief (20 min) compared with ibuprofen tablets (30 min). This finding may be related to differences in the pharmacokinetic profiles of the soluble and tablet formulations of the drug. Indeed, soluble ibuprofen produces an earlier and greater peak plasma concentration of ibuprofen than the tablet form, suggesting that the rate of absorption of ibuprofen is an important determinant of the drug’s efficacy. In agreement with this, Sharma et al. (50) demonstrated, in 50 dental outpatients requiring surgical removal of lower third molar teeth, that the effervescent granule formulation of ibuprofen 600 mg is preferable to the conventional tablet form for managing immediate postoperative dental pain because of its faster onset of analgesic action (Figure 1).
Figure 1.
Mean pain scores in patients treated with ibuprofen 600 mg granules or ibuprofen 600 mg tablets.
Metabolism and excretion
Like other NSAIDs, ibuprofen is extensively metabolised in the liver, principally through cytochrome enzymes P450 2C9 (CYP-2C9), CYP-2C8 and 2C19 participating in the oxidation of the alkyl side chain to hydroxyl and carboxyl derivatives.
Impaired liver metabolism in patients with moderate to severe cirrhosis leads to prolongation of the t1/2 to 3.1 h and 3.4 h for R(−) and S(+) ibuprofen, respectively, with evidence of reduced metabolic inversion of the R(−) to S(+) enantiomer (51). Alcoholic liver disease also prolongs the Tmax as well as the half life of the drug (52).
Phase II metabolism involves formation of phenolic and acyl glucuronides (53) and a minor route of conjugation with taurine which is stereospecific to the S(+) enantiomer because of formation from the thioester CoA which participates in the R(−) to S(+)conversion (54). Biliary excretion in humans of unchanged drug and active phase II metabolites accounts for about 1% of the drug, which compares with the 50% accounted for byurinary excretion (55). The 15 known UDP-gluronyl transferases that catalyse the formation of glucuronides in human liver have been shown to be controlled by five UGTIA and five UGT2B genes and the development of these proceeds from birth to 6 months of age (56).
Ibuprofen shows linear kinetics up to 1200 mg, therefore, within this dosage, the elimination in not saturable. In fact, a dose-dependent efficacy of ibuprofen 400, 600, and 800 mg has been reported in patients with postoperative dental pain (57). Serum concentrations of ibuprofen at 1, 2 and 3 hours after dosing were found to correlate with the global analgesic response.
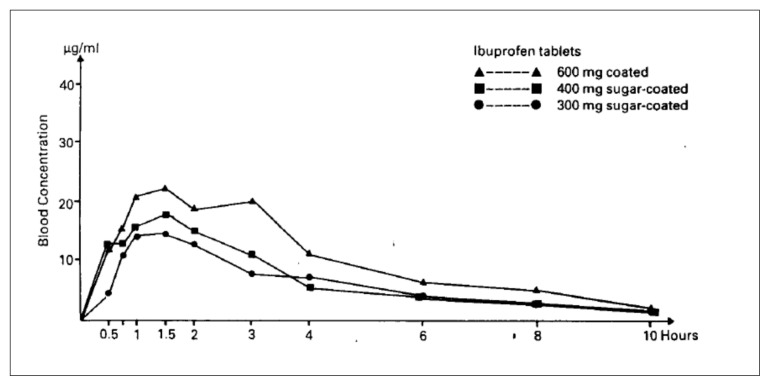
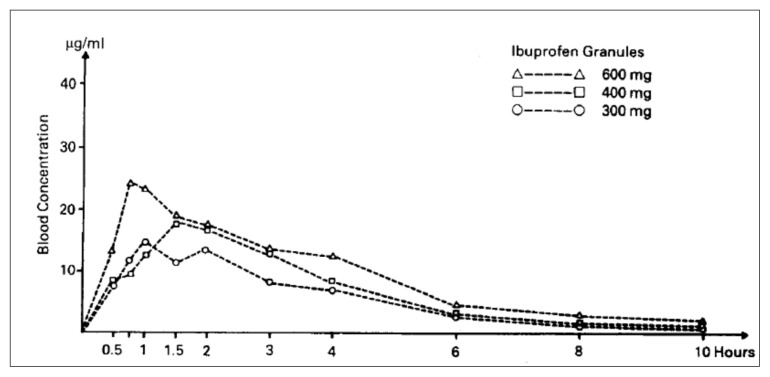
Moreover, a correlation has also been reported between dose and the area under the blood concentration-time curve (AUC), and the high dose is indeed associated with a high AUC; however, the availability of ibuprofen was similar for both the granule and the tablet formulations (58) (Figures 2 and 3).
Figure 2.
Mean plasmatic curves of ibuprofen after single doses of coated tablets [58].
Figure 3.
Mean plasmatic curves of ibuprofen after single doses of granules [58]..
Due to the very short half-life of the drug (2.1 hours), the presence of liver or renal disease does not significantly increase the plasma AUC of ibuprofen and therefore the use of ibuprofen is associated with very low side effects. It is also important to underline that the pharmacokinetic parameters of ibuprofen in children <12 years old can be considered similar to those of young/middle-aged adults (Cmax: 35.8 g/mL; Tmax: 1 to 2 hours; volume of distribution: 0.22 to 0.27 L/kg; half-life 0.9 to 2.3 hours; drug plasma clearance 80 to 110 mL/h/kg; metabolism: CYP2C9 and 2C8).
Clinical implications
Efficacy
Endodontic pain management
Effective pain control is crucial in dentistry, and endodontics is no exception. Pain control particularly during the early phases of endodontic treatment is of paramount importance, and its achievement makes both the dentist and the patient confident and comfortable for the remainder of the treatment (59). Local anaesthesia is the primary method used in dentistry to control patient pain. However, a common clinical problem is the difficulty obtaining satisfactory anaesthesia of an acutely painful inflamed tooth by means of regional block (60). The lack of profound anaesthesia in teeth with inflamed pulp (irreversible pulpitis) is a well-known clinical symptom. It has been suggested that inflammation and infection lower tissue pH, altering the ability of local anaesthetic to provide clinically adequate pain control (61). Others have suggested that the inflammation alters peripheral sensory nerve activity and can lead to inability of local anaesthetic to prevent impulse transmission (62). Other hypotheses are an effect of inflammation on nociceptors or on central sensitisation, and psychological factors (63). Inflamed tissues are associated with a decreased pain perception threshold (59). Thus, tissue that is inflamed is much more sensitive and reactive to a lower stimulus (64). It is assumed that the A sensory fibres could be more responsive in inflamed teeth (65). An effect of inflammation on central sensitisation (activation and sensitisation) is the most likely explanation for the inability of a regional block to achieve profound anaesthesia in mandibular inflamed teeth. Problems in achieving profound pulpal anaesthesia invariably develop in the mandible, particularly in molars and premolars with considerable pulpal inflammation (66). Some studies have reported that a single inferior alveolar nerve lock injection of local anaesthetic (1.8 cc) is ineffective in 30% to 80% of patients with a diagnosis of irreversible pulpitis (67,68,69). Significantly higher amounts of PGs in inflamed compared with normal pulps have been reported (70). Prostaglandins can affect tetrodotoxin-resistant receptors and decrease nerve responses to anaesthetic agents (71,72). Previous investigations have described the anti-inflammatory effects of ibuprofen and indomethacin (73,74). Several subtypes of sodium channels play important roles in mediating inflammatory pain, such as Nan 1.7, Nan 1.8, and Nan 1.9 (75). Prostaglandins play an important role in sodium channel augmentation during inflammation. Pretreatment with ibuprofen prevents up-regulation of the Nan 1.7 and Nan 1.8 sodium channels. Ibuprofen has been used in previous investigations for pre or post-treatment analgesia (76,77). Seymour and Ward-Booth (78) evaluated various doses of ibuprofen (200 mg, 400 mg, and 600 mg) for the management of postoperative dental pain and reported a trend of higher pain relief in patients who had taken 400-mg doses.
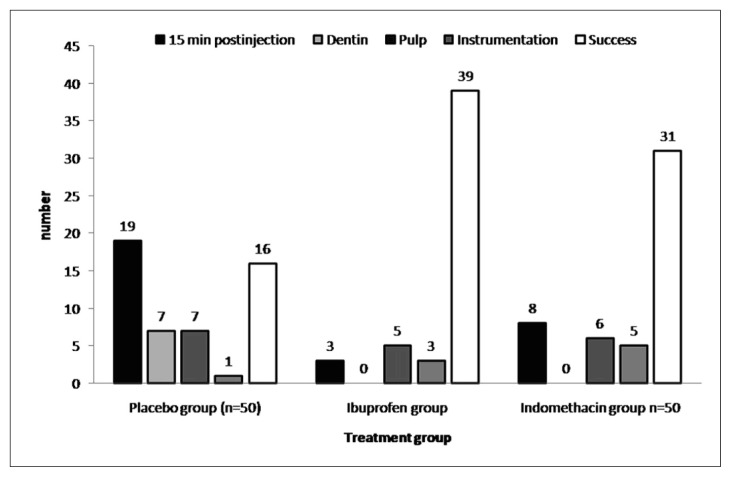
Parirokh reported that premedication with ibuprofen and indomethacin significantly increased the success rates of inferior alveolar nerve block anaesthesia in teeth with irreversible pulpitis. The overall success rates for the placebo, ibuprofen and indomethacin groups were 32%, 78% and 62%, respectively (79). Ibuprofen and indomethacin were significantly better than placebo (p < 0.01). There was no difference between ibuprofen and indomethacin (p = 0.24) (Figure 4).
Figure 4.
Frequency distribution of the stages of failure and success among the three groups (placebo, ibuprofen, and indomethacin) based on patient-reported pain after anesthesia and during access preparation (sample size in each group = 50).
These data are similar to those reported by Ianiro et al. (76), who reported 46.2%, 71.4%, and 76.9% success rates for placebo, acetaminophen, and a combination of acetaminophen with ibuprofen, respectively. Taken together, the results of the above studies suggest that combinations of analgesics are not required and that the use of only one anti-inflammatory drug is just as effective as when combined with acetaminophen.
Indomethacin is an NSAID with strong anti-inflammatory effects that is used for the management of moderate to severe muscular and joint pain. It has not been commonly used or recommended in endodontic therapy and has several side effects that should be considered before it is prescribed (80). In some studies, prophylactic administration of acetaminophen or an NSAID like ibuprofen has been shown to reduce or prevent postoperative dental pain (81). As regards the effects of ibuprofen or acetaminophen pre-medication on the quality of anaesthesia in inflamed teeth during endodontic therapy, Modaresi et al. confirmed that ibuprofen is more effective in achieving profound anaesthesia (77). Ibuprofen premedication increases the depth of anaesthesia because of the COX pathway-blocking and PG-reducing effects of NSAIDs, which result in significant inhibition of stimulated nerve activity (82). Ibuprofen seems to be more effective in achieving a deep anaesthesia than acetaminophen-codeine. The analgesic efficacy of NSAIDs in inflammatory pain has been well established (83). NSAIDs have been shown to be effective for managing pain of inflammatory origin; by virtue of their binding to plasma proteins they actually exhibit increased delivery to inflamed tissue (81). Ibuprofen is vastly superior to acetaminophen-codeine and placebo (77). Preoperative administration of 800 mg ibuprofen 45 minutes before anaesthesia injection in patients with irreversible pulpitis and partial necrosis is recommended in order to ensure profound anaesthesia and a comfortable experience for the patient.
It is well established that, in general, preoperative pain is the main factor determining the level of postoperative pain. One study reported that tooth pain is the most common form of pain in the oral-facial region (84). Another study demonstrated that 20% of patients have moderate to severe post-endodontic pain. NSAIDs, acetaminophen and opioids are active analgesics and can have additive effects when combined. Because opioids and NSAIDs produce analgesia by different mechanisms, the simple additive effect of administering an opioid in combination with an NSAID is often substantially greater than the analgesia achieved by doubling the dose of either drug administered alone (85). Dental pain is a complex process resulting from a combination of biological, biochemical, environmental, and psychogenic factors. Many factors can influence clinicians’ decisions to prescribe analgesics to help combat their patients’ postoperative pain. Currently, there is a gap in the endodontic literature with regard to the question of how particular endodontic diagnoses (surgical and nonsurgical), endodontic procedures, and perceived levels of patient pain might affect the choice of analgesic or combination of analgesics (non-narcotic and narcotic) prescribed. In a recent study it was hypothesised that ibuprofen would be used most often, regardless of the endodontic diagnosis, procedure rendered, or severity of perceived pain: 600 mg ibuprofen given four times per day was found to be the most preferred analgesic prescribed for patients regardless of their perceived level of pain, endodontic diagnosis, or treatment rendered. This result was statistically significant (86). Ibuprofen blocks both the COX-1 and the COX-2 enzymes, but has been shown to be safe and cost-effective with a highly effective analgesic and anti-inflammatory action in post-endodontic pain (87). The prescription of narcotics has gone up in the following conditions: postsurgical pain (28%), postoperative flare-up (31%), or severe pain associated with a necrotic pulp and acute periradicular abscess (34%).
Sutherland and Matthews’ meta-analysis on the effectiveness of interventions used in the emergency management of acute apical periodontitis showed that preemptive NSAIDs in conjunction with pulpectomy provided a significant benefit (88). Endodontic pain is best managed by elimination of the source of the pain, as far as possible, along with judicious use of local anaesthetics and nonopioid or opioid analgesics (89).
Krasner and Jackson noted from their study that although pulpectomy eliminates the source of endodontic pain, postoperative pain and discomfort are fairly common side effects of endodontic treatment, a problem for 25 to 40% of all endodontic patients (90). Placing calcium hydroxide as an intra-canal medication can cause perceived postoperative discomfort for the patient. Several possible reasons why calcium hydroxide may contribute to postoperative pain are: coagulation necrosis, tissue dissolution, cytotoxicity, and bone necrosis. Although postoperative pain may be associated with calcium hydroxide placement, calcium hydroxide actually decreases production of arachidonic acid from membrane phospholipids, thus decreasing levels of prostaglandin E2 and decreasing pain.
Chong and Pitt Ford evaluated the pain experience following root-end resection and filling with mineral trioxide aggregate or intermediate restorative material (IRM). Thirty-seven percent of patients did not take any analgesics following treatment. In order of popularity, the analgesics taken were ibuprofen, acetaminophen, and acetaminophen plus codeine (91). Contrary to Chong and Pitt Ford’s findings, Kvist and Reit noted that significantly more patients reported discomfort after surgical retreatment than after nonsurgical procedures. High pain scores were most frequent on the operative days, whereas swelling peaked on the first postoperative day, after which it progressively decreased both in frequency and magnitude. Analgesics were significantly more often consumed after periapical surgery than after nonsurgical procedures (92). Houck found the majority of patients with symptomatic necrotic teeth had significant postoperative pain and required analgesics to manage this pain (93).
Although combining narcotic analgesics with non-narcotic anti-inflammatory analgesics is beneficial in alleviating postoperative pain, Litkowski et al. found that rates of nausea and vomiting were significantly lower with oxycodone 5 mg/ibuprofen 400 mg compared with oxycodone 5 mg/acetaminophen 325 mg but not with hydrocodone 7.5 mg/acetaminophen 500 mg (95). Keenan et al., considering a Cochrane systematic review, reported that there is no significant difference in pain relief in patients with untreated irreversible pulpitis who received antibiotics versus those who did not (96). The administration of penicillin did not significantly reduce pain, percussion pain, or the number of analgesic medications taken by patients with untreated irreversible pulpitis.
Wisdom tooth extraction
The removal of third molar teeth in a day-case surgery setting has become popular with patients, healthcare trusts and oral surgeons. Management of pain after third molar operations is important, particularly as most patients are treated as day-cases. In addition to alertness and rapid recovery from anaesthesia, well-controlled pain is another indication for discharging a patient home (97).
The trend toward day-case surgery, with discharge on oral medication, has highlighted the need for effective and safe analgesics that facilitate a rapid recovery and discharge time. Postoperative analgesia may be achieved by the use of local anaesthesia or by giving NSAIDS, opioids, or a combination (Table 3).
Table 3.
Efficacy of analgesics after third molar extraction, from systematic reviews of randomised double-blind trials (a total 0f 14,150 patients included in 155 trials of fifteen drug and dose combinations against placebo).
| Number (%) of patients with at least 50% of pain relief | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| Drug and dose | Treatment | Placebo | Relative benefit (95% CI) | Number needed to treat (95% CI) | Total patients | Total trials |
| Valdecoxib 40 mg | 204/279 (73) | 19/194 (10) | 7.3 (4.8 to 11.2) | 1.6 (1.4 to 1.7) | 473 | 4 |
|
| ||||||
| Diclofenac 100 mg | 71/102 (70) | 8/102 (8) | 8.9 (4.5 to 17.5) | 1.6 (1.4 to 1.9) | 204 | 2 |
|
| ||||||
| Valdecoxib 20 mg | 69/101 (68) | 8/103 (8) | 8.8 (4.5 to 17.3) | 1.7 (1.4 to 2.0) | 204 | 2 |
|
| ||||||
| Diclofenac 50 mg | 112/189 (59) | 21/178 (12) | 4.9 (3.3 to 7.5) | 2.1 (1.8 to 2.6) | 367 | 5 |
|
| ||||||
| Rofecoxib 50 mg | 318/557 (57) | 23/262 (9) | 6.6 (4.4 to 9.9) | 2.1 (1.9 to 2.3) | 819 | 6 |
|
| ||||||
| Ibuprofen 400 mg | 1,035/1,835 (56) | 186/1,567 (12) | 4.7 (4.0 to 5.4) | 2.2 (2.1 to 2.4) | 3,402 | 37 |
|
| ||||||
| Ibuprofen 200 mg | 323/695 (46) | 47/499 (9) | 4.6 (3.5 to 6.1) | 2.7 (2.4 to 3.1) | 1,194 | 14 |
|
| ||||||
| Ibuprofen 600 mg | 90/114 (79) | 38/89 (43) | 1.9 (1.5 to 2.5) | 2.8 (2.0 to 4.3) | 203 | 3 |
|
| ||||||
| Celecoxib 200 mg | 39/91 (43) | 4/45 (9) | 4.8 (1.8 to 12.7) | 2.9 (2.1 to 4.8) | 136 | 1 |
|
| ||||||
| Paracetamol 975/1000 mg | 226/616 (37) | 40/422 (9) | 3.8 (2.8 to 5.2) | 3.7 (3.1 to 4.7) | 1,038 | 10 |
|
| ||||||
| Paracetamol 600/650 mg + codeine 60 mg | 217/532 (48) | 64/380 (19) | 2.5 (1.9 to 3.1) | 4.2 (3.4 to 5.5) | 911 | 12 |
|
| ||||||
| Paracetamol 600/650 mg | 224/630 (36) | 76/635 (12) | 2.9 (2.3 to 3.7) | 4.2 (3.6 to 5.2) | 1,265 | 10 |
|
| ||||||
| Aspirin 600/650 mg | 627/1,788 (36) | 255/1,847 (15) | 2.5 (2.2 to 2.9) | 4.7 (4.2 to 5.4) | 3,635 | 46 |
|
| ||||||
| Paracetamol 300 mg + codeine 30 mg | 48/175 (29) | 11/124 (9) | 3.3 (1.8 to 6.2) | 5.4 (3.7 to 9.7) | 299 | 3 |
|
| ||||||
| Dihydrocodeine 30 mg | 8/49 (16) | 2/50 (4) | 4.1 (0.9 to 18) | not calc | 99 | 1 |
Shaded areas are those analgesics used by dentists in the UK
Studying postsurgical dental pain is a sensitive method for evaluating analgesic drugs (98), and the most intense pain occurs after the removal of impacted third molars. Analgesics with an anti-inflammatory action are effective in controlling postoperative dental pain (21) and ibuprofen provides good analgesia after the removal of impacted third molars (99,49). A retrospective analysis of randomised clinical trials conducted over the past 40 years demonstrated that ibuprofen is effective for treating moderate to severe postoperative pain (3,4,5). Numerous studies conducted in patients with postoperative dental pain after third molar surgery confirmed the analgesic effects of ibuprofen in these patients (100,101, 87, 102,103).
Barden and Edwards(104) reviewed the literature on the available analgesics commonly prescribed by dentists, in order to compare the relative efficacy of these drugs after third molar extraction. They collected data from systematic reviews of randomised, double blind studies of analgesics in acute pain, and concluded that NSAIDs and COX-2 inhibitors show the lowest (best) number needed to treat values (NNTs). They may also have fewer adverse effects after third molar surgery,
Sharma (50) compared both the speed of onset and the efficacy of the analgesia produced by the effervescent granule formulation with the speed of onset and efficacy produced by the conventional-release tablet formulation of ibuprofen in patients with acute dental pain requiring surgical removal of unilateral or bilateral lower third molar teeth under general anaesthesia. They also recorded the incidence and severity of any adverse events. In this investigator-blind, parallel-group, multiple-dose study, a total of 50 patients received the effervescent granule formulation of ibuprofen 600 mg (Brufen® granules) as the study treatment and another 50 received the 600 mg tablet formulation. Patients received either one sachet of ibuprofen granules or one tablet of ibuprofen at six-hourly intervals for up to 24 hours once postoperative pain was moderate to severe. Both treatments were shown to be efficacious in treating postoperative dental pain. The granules were found to give significantly better pain relief in the first 30 minutes following the first dose. This may be due to more rapid absorption with the granule formulation in these patients and/or a local action of ibuprofen in solution in the mouth. It was concluded that because of its faster onset of action, the soluble effervescent form of ibuprofen (Brufen® granules) is preferable to the conventional tablet form for managing the immediate postoperative dental pain experienced.
Seymour et al. also reported that a soluble formulation of the drug provided a more rapid onset of analgesia than ibuprofen tablets in patients with early postoperative pain after third molar surgery (49). Differences in efficacy were attributed to earlier and greater peak concentrations of ibuprofen after taking the soluble formulation compared with the tablets. Further investigation showed that both preparations of ibuprofen, soluble and tablet, provided effective pain control in the early postoperative period after removal of impacted third molars, but further medication after 3 hours (when pain intensity is likely to increase) is recommended.
Ahlstrom et al., in a randomised, double-blind, parallel-group trial in 127 adults complaining of at least moderately severe pain after the removal of an impacted third molar, compared the efficacy of single oral doses of drinkable diclofenac dispersible 50 mg with that of ibuprofen 400 mg (an established reference analgesic) and placebo, assessing the onset and duration of pain relief obtained. In comparison with conventional enteric-coated diclofenac sodium, diclofenac dispersible has a rapid onset of absorption and provides a drinkable form of the drug for patients unable or unwilling to swallow tablets. The doses (diclofenac 50 mg and ibuprofen 400 mg) were chosen on the basis of the fact that pain after the removal of an impacted third molar is rather severe; only patients who experienced at least moderately severe pain were eligible for the study. Both diclofenac and ibuprofen produced analgesia within 40 minutes and the effect lasted for up to 6 hours. At 6 hours about 60% of both groups had not taken any rescue medication. In conclusion, diclofenac dispersible is a rapid and effective analgesic for the treatment of postsurgical pain after the removal of an impacted lower third molar, but it is not more effective than conventional ibuprofen (105).
NSAIDs suppress the activity of both isoforms of cyclooxygenase. Inhibition of COX-1, the constitutive isoform, is primarily responsible for the adverse GI effects of this class of drugs, whereas inhibition of COX-2, the inducible isoform, accounts for their therapeutic effects. COX-2 inhibitors such as celecoxib and rofecoxib appear to be as effective as nonselective NSAIDs in the treatment of chronic inflammatory disease, but their analgesic efficacy and safety at the higher doses required for analgesia are less certain. There is consistent evidence that COX-1 plays a major role in the early pain response following injury and that analgesia is increased when both COX-1 and COX-2 are inhibited simultaneously. Early postoperative nociception may cause delayed hyperalgesia by a process of central plasticity. In an experimental model of pain, ibuprofen promptly suppresses PGE2 concentrations, whereas celecoxib has no discernible effect until 90–120 minutes postoperatively, when COX-2 activity is induced. Both drugs significantly reduce pain compared with placebo but celecoxib appears to have a slower onset of action. The analgesic effect of ibuprofen is well characterised for acute pain and short-term treatment is well tolerated (106).
The pre-emptive use of an NSAID before an operation may be more beneficial than its use after an operation (107); opioids, too, are more effective if given before rather than after an operation (108,109,110). Pre-emptive analgesia prevents the establishment of central sensitisation caused by incisional and inflammatory injuries. It starts before incision and covers both the period of the operation and the initial postoperative period (111,112). Postoperative NSAIDs, such as ibuprofen (100,113,114) and paracetamol with codeine (115,116), have been reported to be effective after third molar removal. Basic scientific evidence suggests that an analgesic given before an operation should produce a better outcome than the same drug given after an operation. It is now accepted that the policy of waiting for a patient to report severe pain before prescribing an analgesic results in unnecessary discomfort and may reduce the efficacy of any subsequent treatment (117). Although reviews of clinical findings have been mostly unfavourable (118,119,120,121), there is still a widespread belief in the efficacy of pre-emptive analgesia among clinicians. Three randomised controlled trials with NSAIDs (122,123,124) and one with paracetamol (125) showed no evidence of a pre-emptive effect. In another study, the analgesic efficacy of a single 50-mg preoperative dose of flurbiprofen was compared with ACC-30 (aspirin 375 mg, codeine 30 mg, caffeine 30 mg) and a placebo. The results indicated that better analgesia was obtained when flurbiprofen was given preoperatively compared to only after surgery. Conversely, preoperative administration of ACC-30 did not demonstrate any significant influence on postsurgical analgesia. When comparing the two drugs, flurbiprofen proved to be superior in providing pain relief only when it was given prior to surgery. There was no difference between the drugs when they were given only after surgery. The side effects were moderate and not significantly different between patients receiving flurbiprofen and those receiving ACC-30 (126). A double-blind, randomised cross-over trial was carried out in 50 patients undergoing surgical removal of bilaterally impacted lower wisdom teeth. Paracetamol 1000 mg was administered once preoperatively and once postoperatively. It was concluded that preoperative paracetamol does not offer any clinical advantage in patients who undergo surgical removal of impacted lower wisdom teeth (125). Pre-emptive analgesia is effective in immediate postoperative pain control and there are no significant differences between ibuprofen 600 mg, paracetamol 1g + codeine 60 mg or diclofenac 100 mg (127). In addition, there are no significant differences between the groups with regard to adverse events, including nausea, vomiting, GI discomfort and dizziness. Combinations of paracetamol and codeine have been reported to have more side effects than ibuprofen (119). NSAIDs have a number of side effects (113,114,115,116), including their influence on platelet function. However, the increased risk of bleeding from the perioperative use of NSAIDs is clinically unimportant (118). Careful patient selection and history taking before the use of NSAIDs should avoid any adverse events.
Esteller-Martinez compared the analgesic efficacy of diclofenac sodium versus ibuprofen following surgical extraction of impacted lower third molars. The drug administration protocol was ibuprofen 600 mg every 8 hours and diclofenac sodium 50 mg every 8 hours for 4 days; the rescue medicine was paracetamol/codeine 325/15 mg, two tablets as required. No statistically significant differences in analgesic efficacy emerged between diclofenac sodium and ibuprofen, although the former was associated with an increased need for supplementary medication in the first two postoperative days (128).
Dionne (102) evaluated the analgesic effect of pre and postoperative ibuprofen in outpatients undergoing impacted third molar removal. Patients were given ibuprofen 800 mg prior to the procedure and 400 mg 4 and 8 hours later. Comparison was made with groups receiving either placebo at all doses, 600 mg paracetamol before and 4 and 8 h after surgery, or preoperatively administered placebo followed by two doses of postoperatively administered 600 mg acetaminophen plus 60 mg codeine. The results of this study demonstrated that pretreatment with ibuprofen resulted in a better suppression of postoperative pain when compared to standard therapy without an increase in side effects, suggesting that analgesic drugs that inhibit peripheral prostaglandin synthesis are more efficacious for suppressing postoperative pain than drugs that do not interfere with this pathway (Tab. 4).
Table 4.
Comparison of the analgesic effect of different medications and dosages.
| Drug | Percentage of patients with ≥ 50% pain relief | Number of Patients | Total Trials |
|---|---|---|---|
| Ibuprofen 200 mg | 46 | 1194 | 14 |
| Ibuprofen 400 mg | 56 | 3402 | 37 |
| Ibuprofen 600 mg | 79 | 203 | 3 |
| Diclofenac 50 mg | 59 | 367 | 5 |
| Diclofenac 100 mg | 70 | 204 | 2 |
| Acetaminophen 600/650 mg | 36 | 1265 | 10 |
| Acetaminophen 600/650 mg + Codeine 60 mg | 48 | 911 | 12 |
| Placebo | 12,5 | 6497 | 156 |
Data adapted from Barden, 2004 [129]
Ibuprofen pretreatment followed by postoperative administration of a second dose also resulted in less pain than placebo, a second dose of paracetamol, or the postoperative administration of a standard combination analgesic: paracetamol plus codeine. The greater efficacy of ibuprofen pretreatment in comparison to these standard analgesics suggests that suppression of the processes that contribute to postoperative pain, i.e., the arachidonic acid cascade, results in less pain than the postoperative administration of drugs that attempt to relieve pain by antagonising activated pain pathways. The well-established analgesic effect of ibuprofen 400 mg was confirmed in Averbuch and Katzper’s study, which concluded that the intensity of initial pain is not correlated with the need for larger doses of analgesic (130). In contrast, Laska et al. (57), reported that in the first 0.5 hour the serum level and the clinical efficacy were greater for ibuprofen 600 mg than for the 400 and 800 mg dose., suggesting that the 600 mg dose may have been more bioavailable than the tablets used in the 400 and 800 mg groups. This would help to explain the better analgesic effect of ibuprofen 600 mg at 0.5 hours after administration (57).
Zamiril and Mousavizadeh (131) compared the analgesic efficacy of ibuprofen, celecoxib and tramadol in patients after extraction of mandibular third molar teeth.
Patients were randomly divided into three groups. Group 1 received ibuprofen 600 mg and groups 2 and 3 received celecoxib 200 mg and tramadol 100 mg, respectively, eight hours and one hour before extraction. The patients reported their pain severity in a questionnaire four and eight hours after the tooth extraction.
The maximum severity of pain four hours after tooth extraction in tramadol group was 7, which was greater than the severity recorded in the ibuprofen (4.25) and celecoxib groups. The maximum severity of pain eight hours after tooth extraction in the tramadol group was 8.13, which was also greater than that recorded in the ibuprofen (6.13) and celecoxib (5) groups.
However, it is important to remark that dental patients may experience a delayed response and possible treatment failure when taking ibuprofen for pain relief after surgery for third molar tooth extraction (132). This could be related with the lower doses of ibuprofen used. In fact, has been postulated that the trauma of dental pain and surgery may decreased gastric emptying and secretion mediated perhaps by vagal suppression. This effects is able to reduce the processes of disintegration and dissolution in the stomach with a reduction in the drug absorption (132). In agreement with this hypothesis has been reported that the intramuscular administration of ketorolac is related with an improvement of dental pain, while the oral administration is not better than other NSAIDs such as ibuprofen (133). Moreover a lower efficacy of oral administration of ibuprofen after dental surgery may be also related with an inhibition of chiral inversion of R-ibuprofen to active S-ibuprofen (132). Therefore, it is possible that in the management of oral pain after dental surgery higher doses of ibuprofen (i.e. 600 mg or 800 mg) may be required but the patients may be monitored for the development of side effects.
Pediatric dentistry
Pain management is an important part of dentistry, paediatric dentistry in particular (134). Pain is a common cause of distress in children and its management, despite being the focus of increasing interest during the past decade, is still recognised as frequently being suboptimal (135,136). Perrott et al. (137) summarised studies testing the efficacy and safety of single-dose paracetamol and ibuprofen for treating children’s pain or fever. They found that single doses of ibuprofen (4–10 mg kg−1) and paracetamol (7–15 mg kg−1) have similar efficacy for relieving moderate to severe pain. Although we are improving, very often we are rather poor at providing analgesia to injured children in an adequate and timely fashion. Co-codamol preparations for children (paracetamol/codeine) are seen by many as a “step-up” in the analgesic category –as a moving away from “simple” analgesia such as paracetamol and NSAIDs into the altogether more “potent” world of opiates. Co-codamol may be more difficult to prescribe, as many clinicians feel uncomfortable about discharging patients after its administration. We do not need to move back to co-codamol. Instead, what we need to do is to examine our use of ibuprofen. In North America, the “paediatric dose” of ibuprofen is 10 mg/kg. In the UK, most still use the old 5mg/kg dose, long-since revised by the British National Formulary for Children. The relative safety of short-term use of ibuprofen has been confirmed. Routine anxiety about its use in cases of asthma and fever should have been consigned to history. And while one must always be mindful of dangers such as its potential for renal impairment in those with volume depletion, for the vast majority of cases, it is now time to use 10 mg/kg for children’s pain—the effective analgesic dose of ibuprofen (Tab. 5).
Table 5.
The effective analgesic dose of ibuprofen (Sinifev 20 mg, elixir) in growing children.
| Age | Weight (kg) | Dose | Dose mg/kg (average) | Total Daily Dose mg/kg/day (average) |
|---|---|---|---|---|
| 3 – 6 months | 5.6 – 7.7 | 2.5 ml T.I.D. (150 mg) | 8.9 – 6.5 (7.7) | 26.7 –19.5 (23.1) |
| 6 – 12 months | 7.8 – 10 | 2.5 ml T.I.D. (150 mg) | 6.4 – 5.0 (5.7) | 19.2 – 15.0 (17.1) |
| 1 – 3 years | 11 – 15 | 5 ml T.I.D. (300 mg) | 9.1 – 6.7 (7.9) | 27.3 – 20.1 (23.7) |
| 4 – 6 years | 16 – 20 | 7.5 ml T.I.D. (450 mg) | 9.4 –7.5 (8.5) | 28.2 – 22.5 (25.4) |
| 7 – 9 years | 21 – 28 | 10 ml T.I.D. (600 mg) | 9.5 – 7.1 (8.3) | 28.5 – 21.3 (24.9) |
| 10 – 12 years | 29 – 40 | 15 ml T.I.D. (900 mg) | 10.3 – 7.5 (8.9) | 30.9 – 22.5 (26.7) |
Ibuprofen is at least as effective as acetaminophen with codeine in providing outpatient analgesia for children (62). There is no significant difference in analgesic failure and pain scores, but children receiving ibuprofen have better functional outcomes, specifically play. Children receiving ibuprofen have significantly fewer adverse effects, and both children and parents are more satisfied with ibuprofen. Ibuprofen is preferable to acetaminophen with codeine for out-patient treatment of children (138).
Effective pain management strategies need to be developed for children having dental extractions or undergoing dental decay therapy. Various techniques have been tried to reduce the pain in children following extraction of their teeth, but none have been very effective. These include local anaesthetic infiltration and nerve blockade (139). Local anaesthetic infiltration in children is time-consuming. Moreover, it can lead to a feeling of numbness of the lips and gums, which children may find distressing (140,141).
A topical anaesthetic (0.25% bupivacaine) placed over the socket at the time of extraction did not relieve children’s distress on recovery from general anaesthetic (142). Pre-operative administration of oral analgesics may lessen postextraction pain. A variety of analgesics have been tried in adults. A few studies have evaluated the use of preoperative analgesics in children. Primosch et al. found that there was no significant decrease in post-extraction pain between children in placebo and paracetamol groups (136). Primosch et al. (143) conducted a study of 60 children to evaluate the efficacy of the preoperative administration of ibuprofen and paracetamol compared with a placebo for pain relief after tooth extraction. The preoperative administration of neither analgesic was superior to placebo administration. A study by Pickering et al. (144), however, provided evidence to support the combination of ibuprofen with paracetamol for perioperative analgesia in children after tonsillectomy. McGaw et al. (145) found ibuprofen to be more efficacious than paracetamol or placebo for postoperative pain in children undergoing permanent tooth extraction. Gazal (146) compared the effectiveness of different oral analgesics for relieving pain and distress in children following the extraction of teeth under general anaesthesia. The analgesics studied were paracetamol alone, ibuprofen alone, and paracetamol and ibuprofen in combination. There were significant decreases in the mean pain and distress scores for both the ibuprofen alone and paracetamol/ibuprofen combination groups compared to the control group (usual-dose paracetamol) at 15 min postoperatively. Gazal provided evidence to support the oral administration of ibuprofen alone or in combination with paracetamol for postoperative analgesia in children who are having teeth extracted under general anaesthetic. Ibuprofen and ibuprofen/paracetamol combination were more effective than normal- or high-dose paracetamol at reducing children’s pain and distress following extraction of teeth.
Implant dentistry
Dental implants have become a predictable and widely used treatment for the restoration of oral function (147), as well as for aesthetic improvement (148) in both partially dentate (149) and edentulous patients (150). A successful outcome of dental implantation is largely dependent on preservation of bone support (151). Maintenance of osseointegration and a marginal alveolar bone levels are therefore pivotal for predictable and long-term performance of implant supported prostheses (152). The inhibition of PG production with oral administration of several NSAIDs, including flurbiprofen (153,154), naproxen (155) and meclofenamate sodium (156), can reduce the rate of bone loss associated with periodontal disease. Although it might be tempting to assume that NSAIDs can have a similar influence on the bone supporting dental implants, resulting in a ‘bone sparing effect’ (157), several reports in the medical literature have suggested that NSAIDs can delay bone fracture healing (158,159) and impair bone in growth in orthopaedic implants (160,161). Moreover, many orthopaedic surgeons still recommend the use of NSAIDs after total hip arthroplasty in order to prevent heterotopic ossification (162,163), which is pathological ectopic bone deposition that can limit the motion of the joint, often resulting in functional impairment (164).
In a recent review of the literature (7), it was concluded that the question of the influence of NSAIDs ondental implant osseointegration has not been adequately addressed owing to the lack of prospective studies in humans, and that further research is required to determine whether or not administration of NSAIDs is associated with osseointegration impairment and early failure of dental implants. In short, observations from the orthopaedic literature are possibly one of the main reasons for the reservations, on the part of dental implant surgeons, over the use of NSAIDs for postoperative pain management following implant surgery. Following a pilot study (165), Jeffcoat et al. carried out a randomised clinical trial to evaluate the effect of a three-month course of flurbiprofen (50 mg or 100 mg twice a day) on the marginal bone loss around dental implants in patients. The authors demonstrated a bone-sparing effect with the use of a 100 mg dose of flurbiprofen (157). However, the treatment doses used in the study were consistent with those associated with chronic use and not with those used for postoperative pain relief.
No clinical observation has been published that addresses the question of the effect of the NSAID ibuprofen on the marginal bone around dental implants when the drug is administered postoperatively in a short course at a prescribed dose suitable for pain relief in patients following implant surgery. Therefore, a randomised double-blind placebo-controlled trial was undertaken to ascertain whether the postoperative administration of a one-week course of ibuprofen 600 mg four times daily had an effect on the marginal bone healing and the early osseointegration of dental implants (166). The preliminary results suggested that ibuprofen might not have affected the early bone response to implants placed in the patients recruited in the study. This finding was supported by the lack of statistically significant differences in marginal bone level changes around implants at the 3- and 6-month radiographic assessments following implant placement, between patients who received a short-term therapeutic dose of ibuprofen and those who received placebo.
Ibuprofen is one of the prototypes of the NSAID class of analgesics, which are among the most widely used therapeutics, primarily for the treatment of pain and inflammation following surgical procedures since they are effective in the management of pain, fever, redness and oedema arising as a consequence of inflammatory mediator release (9). Like other drugs of its class, systemic administration of ibuprofen can be associated with side effects. Gastrointestinal upset, bleeding and ulceration are among the most frequently observed side effects of NSAIDs (167). It was noted that the maximum dose of ibuprofen used in the above mentioned study (2.4 g daily divided into four doses of 600 mg each) was well tolerated and also sufficiently effective for pain relief following implant placement, as suggested by the infrequent use of the rescue analgesic by the ibuprofen group patients.
Prostaglandins are considered potent mediators of inflammation (168). It has been experimentally demonstrated that, during the inflammatory process, levels of PGs are increased in a wide range of inflamed tissues including gingival tissues (169). Furthermore, elevation of PGs, particularly of PGE2 in crevicular fluid, is often associated with a localised bone resorption activity (170,171). Since the mechanism of action of the NSAIDs involves inhibition of conversion of arachidonic acid to the PG series of metabolites, it is reasonable to hypothesise that inhibiting conversion of arachidonic acid to prostaglandins by NSAIDs could result in a bone-sparing effect such as that observed in several reports in the dental implantology (157,165) and periodontology literature (154,155,156,157). However, the evidence for this is by no means conclusive because PGs, particularly the E-type produced by osteoblasts under physiological or pathological conditions, have bimodal functions during the bone remodelling process and may also promote bone formation (172). In addition, it was demonstrated in an experimental rat model that low levels of pharmacological doses of PGs such as PGE2 may stimulate bone formation in the mandible (173). Thus, these data may be used to support the hypothesis that the administration of NSAIDs could have an inhibitory effect on bone healing around implants, because these compounds inhibit PG formation, as has been shown in the orthopaedic literature. However, such studies were performed in animal models using different doses dissimilar to those used in humans, which could lead to contradictory conclusions, particularly if their results are applied to bone biology and osseointegration concerns in humans.
Short-term use of ibuprofen of 200 mg or 400 mg taken three times daily has been shown to cause a considerable reduction in total body synthesis of the PG type-E in healthy humans (174). Moreover, Kehoe et al. demonstrated a 21-fold reduction in PGE2 production in a sample of inflammatory exudate from the periodontal ligament space when ibuprofen was administered in a 30 mg/kg dose twice daily in an experimental study on the effect of ibuprofen on orthodontic tooth movement. The authors suggested that ibuprofen may reduce bone resorption as measured by tooth movement (175).
Ibuprofen used as a post-operative analgesic (1-week course of 600 mg of ibuprofen taken four times daily) may not have a significant negative impact on marginal bone level around dental implants (166).
Orthodontic pain management (176)
No matter how much progress has been made in orthodontics or how competent the practitioner is, orthodontic treatment is still associated with discomfort. Pain and discomfort are common clinical symptoms in orthodontic patients, especially 2 to 4 days after the placement of fixed orthodontic appliances. It has even been suggested that orthodontic pain can discourage some patients from seeking treatment and might cause a number of patients to discontinue treatment (177). After an orthodontic procedure, it is typical to experience pain and soreness 24 hours after placement of the appliance. The pain generally arises following placement of the first archwire (178, 179, 180) and subsides after a further week (181). Orthodontic appliance and treatment acceptance can be predicted by the degree of initial pain and discomfort. The more pain associated with initial orthodontic treatment, the less compliant the patient will be during treatment.
Researchers attributed both the initial and delayed pain response to hyperalgesia of the periodontal ligament. This hyperalgesia makes the periodontal ligament sensitive to algogens that are released, such as histamine, bradykinin, PGs, and serotonin (182). The increase in the levels of these mediators elicits a pain response following orthodontic force application. Tooth movement is a complex phenomenon, and various studies have attempted to explain its mechanism. According to the pressure-tension theory, tooth movement occurs in three stages: alterations in blood flow associated with pressure in the periodontal ligament (PDL), formation or release of chemical messengers, and activation of cells (183). Prostaglandin (PG) E and interleukin-1 B levels increase in the PDL and the gingival crevicular fluid within a short time after the application of pressure and appear to be important cellular response mediators, working by increasing the number of multinuclear osteoclasts, osteoclastic bone resorption, and the rate of orthodontic tooth movement. Several studies demonstrated that the application of PG-E1 or 2 resulted in increased tooth movement in both rats and humans, emphasising its important role in the mechanism of tooth movement (184,185,186,187).
Inflammatory mediators, such as PG 1 or 2, contribute to orthodontic tooth movement and are also involved in the mediation of orthodontic pain. NSAIDs that block PG production are commonly given to patients for pain relief.
At present there is no universal recommendation on the use of analgesics in pain reduction. NSAIDs such as ibuprofen and acetaminophen are commonly recommended. Their analgesic action has been explained by their ability to inhibit the synthesis of PGs at the site of the tissue injury. This is thought to occur through inhibition of the cyclooxygenase enzymes COX-1 and COX-2 (188). Ibuprofen has been considered a representative NSAID on the basis of its efficacy for postoperative relief of dental pain, while acetaminophen has been believed not to affect tooth movement, and aspirin has been considered the traditional NSAID. The question of whether ibuprofen offers an advantage in terms of pain relief compared with acetaminophen and aspirin needs to be further studied.
A systematic review by Xiaoting et al. (176) compared the clinical outcome of different methods of pain intervention. Two questions were asked:
Are medications still the main treatment modality to reduce orthodontic pain?
Are there any other new approaches that have been proved to be more effective in pain control?
Since gastric ulceration, bleeding disorders, allergy, etc., are among the common adverse effects of NSAIDs, orthodontic researchers and clinicians have focused on the search for much safer analgesics among the many kinds of NSAID. Initially, ibuprofen was highlighted as safe and effective. However, there are still many controversies over the use of NSAIDs because of their potential influence on tooth movement (189,190). Acetaminophen is preferred because it does not inhibit PG synthesis and has no deleterious effects on tooth movement (191,192,193). A meta-analysis has revealed that there is no difference in pain relief between ibuprofen, acetaminophen, and aspirin, although compared with a placebo, ibuprofen has a better effect on pain control (176). Other studies comparing the efficacy of ibuprofen and acetaminophen found statistically significant differences (194). In their study of impacted third molar removal, Dionne et al. found that ibuprofen resulted in significantly less reported pain than a placebo or acetaminophen taken before the procedure and administered 4 and 8 hours later (102). Forbes et al. also found similar results in a study of surgical removal of impacted third molars (195). They concluded that 400 mg of ibuprofen provided superior pain relief compared with acetaminophen alone or in combination with codeine. Cooper concluded from his five studies on ibuprofen for postsurgical pain that ibuprofen 400 mg is consistently more effective than aspirin 650 mg, acetaminophen 600 mg, and aspirin and acetaminophen combined (196). Most of these studies concluded that ibuprofen provided significantly faster and greater relief than did acetaminophen. Although these findings contradict the results on reducing orthodontic pain, which show no statistically significant differences between ibuprofen, acetaminophen, and aspirin (176), they might simply highlight the great differences between postsurgical pain and the much less severe orthodontic pain. Less pain is experienced with an orthodontic appliance if ibuprofen 400 mg is taken 1 hour before, 3 hours after, and 7 hours after placement. Preemptive and post-treatment ibuprofen administration reduced orthodontic pain significantly at 6 hours and at bedtime on the night of the orthodontic procedure. A rebound effect and high pain ratings at 24 hours indicate that additional doses should be given after the 7-hour dose to maintain the benefits of the medication (197).
Analgesics are still the main treatment modality to reduce orthodontic pain. However, the pharmacological actions as well as their side effects should be identified before prescribing these medications in routine clinical practice. Some long-acting NSAIDs and COX-2 inhibitors, such as ibuprofen, are interestingly recommended on the strength of their comparatively few side effects, and their pre-emptive use is promising. The downside of NSAIDs is that they inhibit PG synthesis and therefore delay or inhibit orthodontic tooth movement. Although much has been published on this subject, it is still controversial. Some clinicians argue that, since lower doses of these medications are used in humans and for shorter durations, in a healthy subject they are cleared by the body before tooth movement and therefore have no effect on tooth movement. Other relatively safer approaches such as low level laser therapy (LLLT) have aroused researchers’ interest. To date, however, there is still only limited evidence suggestive of benefits of LLLT, vibratory stimulation, and other non-pharmacological modalities.
Periodontal pain management
Chronic periodontitis is a common inflammatory disease of the gums and related bones (198).
Periodic professional mechanical plaque removal is a standard procedure listed under internationally recognised “parameters of care” to control chronic periodontitis and to maintain periodontal health, although its efficacy on the prevention of periodontal diseases is currently debated (199,200). Pain or discomfort is often associated with non-surgical plaque removal (201,202,203,204). The ideal anaesthetic agent is characterised by convenient and painless administration, fast onset, adequate duration, and minimal adverse effects. NSAIDs meet most of these criteria and their efficacy for dental surgery pain is well established. There is no evidence that any nonselective NSAID is more effective than another for non-specific pain management, but ibuprofen is nowadays considered the safest inexpensive choice (205). The addition of arginine to ibuprofen enhances the rate and extent of absorption of ibuprofen so that ibuprofen arginine becomes bioavailable about three times more rapidly than generic ibuprofen (206). Ibuprofen arginine, because of its rapid onset of action and long duration, its favourable safety profile and the possibility of easy oral administration shortly before a dental procedure, is a promising agent to achieve pain control during and after periodontal scaling and root planing (SRP) (206,207). For patients with mild to moderate chronic adult periodontitis treated in a general dental practice, a single dose of ibuprofen arginine 800 mg taken 30 minutes before treatment proved to be an effective and safe medication for maximising comfort during treatment, reducing average and maximum pain levels during SRP compared with placebo (208).
Tooth whitening
Gingival irritation and tooth sensitivity are the most common side effects of vital tooth bleaching. (209). One clinical study, for example, showed that 55% of patients treated with carbamide peroxide reported tooth sensitivity and 20% of those who experienced side effects terminated treatment due to discomfort (210).
Charakorn and Cabanilla studied the effects of ibuprofen 600 mg on tooth sensitivity from in-office bleaching with 38% hydrogen peroxide. They performed a double-blind randomised controlled trial on 30 patients retaining all anterior teeth. They measured the level of tooth sensitivity by using a modified visual analogue scale. The authors observed that ibuprofen 600 mg (single dose) decreased tooth sensitivity associated with in-office bleaching only during treatment time. This finding suggests that ibuprofen 600 mg single dose may be used to help patients who have a lower pain threshold get through the treatment(211).
Safety
Adverse drug reactions
Gastrointestinal toxicity
The long-term use of classical NSAIDs is related to the development of adverse drug reactions such as gastric toxicity, which has precluded a wider extension of their therapeutic use (212). In fact, PGE2 and prostacycline are both hyperalgesic (elicit an increased sense of pain) and gastroprotective. Thus, nonselective COX inhibition with agents such as aspirin, ibuprofen, indomethacin and naproxen, which inhibit both COX-1 and COX-2 enzymes, provides effective pain relief for inflammatory conditions but carries with it a risk of erosive gastritis and GI bleeding. In view of the relative paucity of COX-2 expression in the GI tract and the relative abundance of COX-2 expression in inflamed and painful tissues, selective COX-2 inhibitors (valdecoxib, rofecoxib, celecoxib, and others still in development) were developed to minimise GI toxicity.
Previously we reported in a retrospective study that NSAIDs caused >55% of the adverse drug reactions detected in hospitalised patients, which are common in subjects aged>61 years. Moreover we reported that the adverse drug reactions induced by NSAIDs affected the skin, GI tract and respiratory system and that the drugs more commonly involved were diclofenac and aspirin (213). A systemic review of studies that examined the relative risks of GI complications associated with different NSAIDs found ibuprofen to be the least toxic NSAID (214). According to Lugardon et al., the reported risk of GI events was low among patients treated with ibuprofen, compared with diclofenac, naproxen, ketoprofen, celecoxib, piroxicam (215). Moreover, Moore described that during NSAID treatment, significant GI adverse effects were more common with aspirin (7.1%) and acetaminophen (5.3%) than ibuprofen (4%) (216). Lower rates of occurrence of GI complications in patients treated with ibuprofen could be attributed to its short half-life (about 2 hours). Thus, there is a good pharmacokinetic rationale to account for the low rate of GI adverse drug reactions with ibuprofen.
Liver toxicity
Several papers have described fatal hepatotoxicity in patients receiving both conventional NSAIDs and selective COX-2 inhibitors, e.g. diclofenac, nimesulide, celecoxib, lumiracoxib (217,218,219), as well as acetaminophen (220).
Moreover, we also reported that nimesulide is able to induce livertoxicity probably through the hepatic bioactivation of nimesulide. Indeed, hepatic bioactivation of nimesulide produces reactive metabolites that have the potential to induce intracellular oxidative stress and mitochondrial injury. (221). Thus, acetaminophen use could be related to dose-dependent development of liver toxicity (222), and the daily dose should be lower than 4g, as indicated by the FDA (223). At higher doses, acetaminophen is metabolised by CYP2E1 into a toxic metabolite (N-acetyl-p-benzoquinoneimine) (224) that, reducing the detoxification system of glutathione, is able to induce hepatocyte death.
In contrast, hepatic reactions are probably rarely associated with ibuprofen. Since there have been no specific reports of hepatic reactions with OTC use of ibuprofen, either in trials (225, 226) or in literature analyses (226), it is likely that hepatotoxicity is not a significant risk factor at OTC dosages.
In fact Italian data (227) documented that the percentage of patients with liver toxicity during NSAID treatment is very low during treatment with ibuprofen (1.4) versus other NSAIDs (diclofenac 2.8; ketorolac: 4.6; nimesulide 13.8).
Cardiovascular safety
NSAIDs and coxibs are likely to induce serious cardiovascular events. In the cardiovascular system, prostacycline derived from the metabolism of arachidonic acid, is the dominant prostanoid produced by endothelial cells and it is able to regulate complex interactions between platelets and the vessel wall, antagonising aggregation through the binding with platelet IP receptors (228,229). Platelets contain only COX-1, which converts arachidonic acid to the potent proaggregatory, vasoconstrictive eicosanoid thromboxane A2 (TXA2), the major COX product formed by platelets. Non-selective COX inhibition with aspirin is effective on arterial thrombosis because of its ability to reduce COX-1-dependent production of platelet TXA2; by contrast, selective inhibition of COX-2 (rofecoxib and celecoxib) could produce a relative reduction in endothelial production of prostacycline, while leaving the platelet production of TXA2 intact, increasing the risk of thrombotic cardiovascular events (230). In particular, cardiovascular events including myocardial infarction and hypertension were noted particularly with rofecoxib (231).
Chou et al., reported that serious coronary heart disease incidence rate ratios were much higher for rofecoxib (RR, 2.29; 95% CI, 1.24–4.22; p=0.008) at a more than 25 mg dose than for celecoxib (RR, 1.61; 95% CI, 1.01–2.57; p=0.046) at a more than 200 mg dose (232).
However, celecoxib is also able to significantly increase the risk of cardiovascular events in a dose-dependent manner (233). COX-2 inhibitors may increase cardiovascular risk at high doses through the activation of thrombosis via decreased PGI2 production in the endothelium and unchecked production of TXA2 by COX-1. The imbalance in circulating levels of PGI2 and TXA2 results in increased vascular tone, platelet aggregation, and vascular smooth muscle proliferation due to the unopposed TXA2 effects (234). No conclusive data concerning cardiovascular safety was described during acetaminophen treatment. In fact, Curhan (235) and Chan (236) reported an increase in CV events in women treated with acetaminophen; however, this increase was the same as with common NSAIDs (RR1.35 and RR 1.44, respectively).
Conversely, ibuprofen seems to carry a low risk of cardiovascular events and Rahme and Nedjar (237) showed the following adjusted hazard ratios: ibuprofen 1.05 (0.74–2.41), diclofenac 1.69 (1.35–2.10), naproxen 1.59 (1.31–1.93), celecoxib 1.34 (1.19–1.52), rofecoxib 1.27 (1.13–1.42) and acetaminophen 1.29 (1.17–1.42).
In addition, Troughton and coworker documented that ibuprofen could represent the first line in treatment of fever, pericardial pain, and inflammation in patient with uncomplicated pericarditis because it show a good safety profile and also because it can be titrated across a range of doses (238).
In agreement with these data, recently, at the European Society of Cardiology (ESC) 2010 Congress, it was reported that in people living in Denmark, NSAID use was associated with an increased risk of stroke, ranging from about 30% with ibuprofen and naproxen to 86% with diclofenac (see Table 6).
Table 6.
Risk of stroke with several NSAIDs (European Society of Cardiology ESC - 2010 Congress).
| NSAID | HR (95%CI) for risk of stroke |
|---|---|
| Ibuprofen | 1.28 (1.14–1.44) |
| Diclofenac | 1.86 (1.58–2.19) |
| Rofecoxib | 1.61 (1.14–2.29) |
| Celecoxib | 1.69 (1.11–2.26) |
| Naproxen | 1.35 (1.01–1.79) |
NSAIDs and bone
COX-1 is expressed in normal bone, while COX-2 is up-regulated during bone repair and in the presence of several stimuli such as inflammation. In particular, has been reported that PGE2 is able to induce the resorption during inflammatory diseases (239).
However no definitive data have been reported in experimental models regarding the effects of conventional non-selective NSAIDs (ibuprofen, naproxen and ketorolac) on long bone fracture healing. In fact while Radi et al. (240) reported inhibitory effects on long bone fracture healing, other authors failed to document such effects (241).
As reported for conventional NSAIDS, there are several controversies surrounding coxib (242,243).
NSAIDs and drug interactions
Displacement to plasma proteins
Free NSAID concentrations (i.e. those non-bound to albumin) are generally regarded as pharmacologically relevant to the actions of these drugs, as well as to the untoward effects of drug-drug interactions, where toxic effects of NSAIDs or other drugs are due to displacement of one or other from the albumin or other plasma proteins. As with many NSAIDs, most of which bind to plasma proteins (around 99%), ibuprofen also strongly binds to albumin (244). In particular as reported in Table 7, ibuprofen binds to site II (benzodiazepine) of albumin, while salicylates, diclofenac and naproxen bind to site I (warfarin).
Table 7.
Drugs binding to site I (warfarin) or II (benzodiazepines) of albumin.
| Site I (warfarin) | Site II (benzodiazepine) |
|---|---|
| Chlorothiazide | Ketoprofen |
| Phenytoin | Ibuprofen |
| Glibenclamide | Indomethacin |
| Naproxen | Dicloxacillin |
| Salicylates | Nimesulide |
| Nimesulide | |
| Diclofenac | |
| Sulphamidics | |
| Fluoroquinolones | |
| Valproate |
Therefore, diclofenac is more likely to show a drug-drug interaction with warfarin than with ibuprofen. This is in agreement with recent guidelines suggesting a treatment with ibuprofen in patient chronically treated with warfarin.
Liver metabolism
Inhibition of CYP-2C8 by administration of gemfibrozil to humans increases the plasma concentrations of R(−)-ibuprofen by about one third, as well as prolonging the elimination half-lives of R(−) and S(+) by 54 and 34%, respectively, and increasing AUC values by about 20% (245). All this suggests that CYP-2C8 plays a major role in oxidative metabolism of the ibuprofen enantiomers.
However, there are at present no data concerning inhibitory effects of ibuprofen on CYP enzymes. By contrast, it has been well documented that celecoxib is an important inhibitor of CYP-2D6 and increases the area under the serum concentration-time curve (AUC) of metoprolol (about 64%) (246).
Renal excretion
Several reports suggest that NSAIDs are able to inhibit the renal excretion of digoxin, lithium and tacrolimus (45,247,248).
Moreover, Igbal and coworkers documented that diclofenac induces an increase in the plasma AUC of ciprofloxacin while reducing the total body clearance (249).
As documented by Karjalainen et al.(250), diclofenac is not a CYP inhibitor, but it does induce a dose-dependent inhibition of OAT-1-4 pumps involved in renal excretion (251). With this mechanism, other authors documented that diclofenac is able to increase the rosuvastatin plasma concentration (252). Moreover, diclofenac and salicylates are also able to increase the plasma concentration of metothrexate through competition with the excretion on the MRP 2 and 4 renal pumps.
Aspirin-NSAID interactions
Previously, Catella-Lawson et al. (253) documented in healthy patients that ibuprofen may interfere with the anti-platelet effects of aspirin. In fact, the authors treated healthy patients with aspirin 81 mg taken 2 hours before ibuprofen 400 mg each morning for 6 days and then evaluated the synthesis of prostaglandins. The authors documented that when aspirin was given either before or after ibuprofen, there was complete inhibition of the effect of aspirin on serum thromboxane and platelet aggregation. The decrease in both platelet aggregation and thromboxane production during ibuprofen treatment was not evident during paracetamol, diclofenac or rofecoxib treatment. By contrast, Kimmel et al. (254) reported that in patients with no history of coronary artery disease the use of aspirin was associated with a lower risk of myocardial infarction, as expected, but this benefit was not seen in patients who took any NSAID in addition to aspirin. Patients with established coronary disease who used aspirin with NSAIDs had a similar risk of developing myocardial infarction compared to patients who had taken aspirin alone. Moreover, in elderly patients, with a history of myocardial infarction the mortality of those who had received aspirin and a non-steroidal drug was similar to that of patients who had been prescribed aspirin alone (255,256). No apparent differences were observed in the mortality in patients who had been prescribed aspirin and ibuprofen compared with those prescribed aspirin alone (255). Moreover, Cryer et al. showed that prior treatment for 8 days with aspirin is not affected by subsequent ibuprofen treatment in terms of platelet thromboxane production (257).
By contrast, recently Schujit et al.(258) reported in healthy volunteers more thrombotic cardiovascular events (2.14%) during ibuprofen/aspirin therapy than in patients using lumiracoxib combined with aspirin (0.25%; p< 0.03), even though no difference was observed in a subgroup using ibuprofen or lumiracoxib only (0.92% vs. 0.80% respectively). Therefore, these authors suggest that diclofenac should be preferred to ibuprofen for combined use with aspirin. Conversely, in 2007, the FDA stated on its MedWatch website (259) that with concomitant use of ibuprofen and aspirin there is likely to be a minimal risk in the attenuation of the anti-platelet effects during the treatment with low-dose of aspirin. Moreover, they state that patients who use immediate-release aspirin (not enteric-coated) and take a single dose of ibuprofen 400 mg should take the dose of ibuprofen at least 30 minutes or longer after the aspirin to avoid attenuation of the effect of aspirin on platelets. Therefore, on the basis of information of the FDA and the available published literature it is clear that separation of the dose of aspirin from that of ibuprofen is a practical means of avoiding the potential for impairment of the anti-platelet effect of aspirin by ibuprofen. It should be noted that in an earlier study in patients with rheumatoid arthritis, Grennan et al. (260) showed that high-dose aspirin (3.6 g day-1), but not a lower dose of 2.4 g day-1, in combination with high- or low-dose ibuprofen had a weak clinical additive effect on indices of articular function and pain and this appeared to be related to an increase in serum ibuprofen by aspirin, but ibuprofen administration did not affect serum salicylate levels. Thus, high doses of aspirin (not those usually used for anti-thrombotic effects) may have some impact on the clinical efficacy of ibuprofen in a positive sense, but this is related to effects on ibuprofen concentration in the plasma.
Antihypertensive drugs
Previously, a negative interaction has been reported between NSAIDs and antihypertensive therapy. However, in a study in stage 1 and 2 hypertensive patients on low and high sodium diets receiving the angiotensin-converting enzyme (ACE) inhibitor, enalapril, ibuprofen 1,200 mg daily did not affect systolic or diastolic blood pressure although in a related study indomethacin reduced the effects of capropril (261). Other NSAIDs are well-known to interfere with the actions of ACE inhibitors (262). Conversely, inhibition of the renin-angiotensin system up-regulates COX-2 (263) and thus may exacerbate NSAID-related renal functions. Calcium channel blockers do not appear to be affected by ibuprofen and other NSAIDs in hypertensive patients (264).
Conclusions
Alleviating pain is of the utmost importance when treating dental patients, as it is prevalent and has far-reaching implications, for both the patient and the clinician. The major cause of pain is thought to be the release of inflammatory mediators that activate sensory nociceptors surrounding the tooth. Ibuprofen has one of the best safety profiles of the nonselective NSAIDs, particularly at OTC doses. The faster onset of effect shown by ibuprofen compared to celecoxib reduced the need for early re-medication with the rescue analgesic. The approved OTC dose regimen of ibuprofen (400 mg/6h) has been found to provide significantly longer relief than the maximum daily dose approved for celecoxib (200 mg once a day). Pain control is crucial in dentistry, and endodontics is no exception. Local anaesthesia is the main method used in dentistry to control patient pain. However, a common clinical problem is the difficulty obtaining satisfactory anaesthesia of an acutely painful inflamed tooth by means of a regional block. The lack of profound anaesthesia in teeth with inflamed pulp (irreversible pulpitis) is a well-known clinical symptom. Ibuprofen pre-medication therapy increases the depth of anaesthesia because of the COX pathway-blocking and PG-reducing effects of NSAIDs, which result in significant inhibition of stimulated nerve activity. Ibuprofen seems to be more effective in achieving a deep anaesthesia than acetaminophen-codeine.
Moreover, respect to acetaminophen, ibuprofen administration is rarely associated with liver toxicity. In fact in both trials (225, 226) and literature analyses (226), hepatotoxicity don’t represents a significant risk factor at OTC dosages.
Premedication with ibuprofen and indomethacin significantly increased the success rates of inferior alveolar nerve block anaesthesia in teeth with irreversible pulpitis, with no difference emerging between ibuprofen and indomethacin. Indomethacin is an NSAID with strong anti-inflammatory effects that is used for the management of moderate to severe muscular and joint pain. It has several side effects that should be considered before it is prescribed for dental pain management. Ibuprofen 600 mg given four times per day was found to be, statistically significantly, the most preferred analgesic prescribed for patients, irrespective of their perceived level of pain, endodontic diagnosis, or treatment rendered. Ibuprofen blocks both the COX-1 and the COX-2 enzymes, but has been shown to be safe and cost-effective with a highly effective analgesic and anti-inflammatory action in postendodontic pain. Studying post-surgical dental pain is a sensitive method for evaluating analgesic drugs, and the most intense pain occurs after the removal of impacted third molars. Numerous studies conducted in patients with postoperative dental pain after third molar surgery confirmed the analgesic effects of ibuprofen. Comparing both the speed of onset and the efficacy of the analgesia produced by the effervescent granule formulation (Brufen® granules 600 mg) with the speed of onset and efficacy produced by the conventional-release tablet formulation in patients with acute dental pain at six-hourly intervals for up to 24 hours, both treatments were shown to be efficacious in treating post-operative dental pain. The granules were found to give significantly better pain relief in the first 30 minutes following the first dose. This may be due to more rapid absorption with the granule formulation in these patients and/or a local action of ibuprofen in solution in the mouth. It was concluded that because of its faster onset of action, the soluble effervescent form of ibuprofen (Brufen® granules) is preferable to the conventional tablet form for managing the immediate postoperative dental pain experienced.
It has been reported that a soluble formulation of the drug provided a more rapid onset of analgesia than ibuprofen tablets in patients with early postoperative pain after third molar surgery. Differences in efficacy were attributable to earlier and greater peak concentrations of ibuprofen after taking the soluble formulation compared with the tablets. Further investigation showed that both preparations of ibuprofen, soluble and tablet, provided effective pain control in the early postoperative period after removal of impacted third molars, but further medication after 3 hours (when pain intensity is likely to increase) is recommended.
Diclofenac dispersible 50 mg is a rapid and effective analgesic for the treatment of postsurgical pain after removal of an impacted lower third molar, but it is not more effective than conventional ibuprofen 400 mg (105). Both diclofenac and ibuprofen produced analgesia within 40 minutes and the effect lasted for up to 6hours. Comparing diclofenac sodium with ibuprofen following surgical extraction of impacted lower third molars, there emerged no statistically significant differences in analgesic efficacy between diclofenac sodium (50 mg every 8 hours for 4 days) and ibuprofen (600 mg every 8 hours for 4 days), although the former was associated with an increased need for supplementary medication in the first two postoperative days (128).
The pre-emptive use of an NSAID before operation may be more beneficial than its use after an operation. Pre-emptive analgesia is effective in immediate postoperative pain control and there emerged significant differences between ibuprofen 600mg, paracetamol 1g + codeine 60mg or diclofenac 100mg, even though combinations of paracetamol and codeine have been reported to have more side effects than ibuprofen.
Moreover, respect to diclofenac, ibuprofen shows lower rates of GI complications (215) and a better cardiovascular safety (237), with low risk of drug-drug interactions.
Evaluation of the analgesic effect of pre and postoperative administration of ibuprofen in outpatients undergoing impacted third molar removal confirmed that pretreatment with ibuprofen results in a suppression of postoperative pain. Ibuprofen pre and post-treatment resulted in significantly less pain than pretreatment with paracetamol and paracetamol plus codeine. This suggests that analgesic drugs that inhibit peripheral PG synthesis are more efficacious for suppressing postoperative pain than drugs that do not interfere with this pathway.
The greater efficacy of ibuprofen pretreatment in comparison to these standard analgesics suggests that suppression of the processes which contribute to postoperative pain, i.e., the arachidonic acid cascade, results in less pain than the postoperative administration of drugs that attempt to relieve pain by antagonising activated pain pathways.
The well-established analgesic effect of ibuprofen 400 mg was confirmed in Averbuch and Katzper’s study, which concluded that the intensity of initial pain is not correlated with the need for larger doses of analgesic. The basic pharmacokinetic properties of ibuprofen have been well studied. It is reported to be rapidly absorbed, reaching a mean peak serum level at between 1.5 and 2 hours. Increased ibuprofen serum levels lead to increased analgesia. The mean analgesic scores provided little or no evidence of a dose-response relationship in terms of clinical efficacy when considering the 400 and 800 mg doses. The formulation of the 400 mg tablet was, however, different from that of the 600 mg tablet. Differences in formulation could very well influence the rate of absorption and, in turn, the clinical response. The data suggested that the 600 mg dose may have been more bioavailable than the tablets used in the 400 and 800 mg groups. This would help to explain the better analgesic effect of the ibuprofen 600 mg at 0.5 hours after administration (57).
Pain management is an important part of dentistry, and paediatric dentistry in particular. Pain is a common cause of distress in children, and its management, despite being the focus of increasing interest during the past decade, is still recognised as frequently being suboptimal. The “paediatric dose” of ibuprofen is 10 mg/kg and it is at least as effective as paracetamol with codeine in providing analgesia for children. Children receiving ibuprofen have significantly fewer adverse effects, and both children and parents are more satisfied with ibuprofen. The oral administration of ibuprofen alone or in combination with paracetamol is effective for postoperative analgesia in children who are having teeth extracted under general anaesthetic. Ibuprofen and ibuprofen/paracetamol combination were more effective than normal- or high-dose paracetamol in reducing children’s pain and distress following extraction of teeth.
Dental implants have become a predictable and widely used treatment for restoration of oral function. Maintenance of osseointegration and marginal alveolar bone levels are therefore pivotal for predictable and long-term performance of implant-supported prostheses Ibuprofen used as a postoperative analgesic (1-week course of 600 mg of ibuprofen taken four times daily) may not have a significant negative impact on marginal bone level around dental implants. Pain and discomfort are common clinical symptoms in orthodontic patients, especially 2 to 4 days after fixed orthodontic appliances are placed. It has even been suggested that orthodontic pain can discourage some patients from seeking treatment and might cause a number of patients to discontinue treatment. Less pain is experienced with orthodontic appliances if 400 mg of ibuprofen is taken 1 hour before, 3 hours after, and 7 hours after placement. Pre-emptive and post-treatment ibuprofen administration significantly reduced orthodontic pain. A rebound effect and high pain ratings at 24 hours indicate that additional doses should be given after the 7-hour dose to maintain the benefits of the medication. The downside of NSAIDS is that they inhibit PG synthesis and therefore delay or inhibit orthodontic tooth movement. Although much has been published on this subject, it is still controversial. Some clinicians argue that, since lower doses of these medications are used in humans and for shorter durations, in a healthy subject they are cleared by the body before tooth movement and therefore have no effect on tooth movement.
Chronic periodontitis is a common inflammatory disease of the gums and related bones.
Periodic professional mechanical plaque removal is a standard procedure listed under inter-nationally recognised “parameters of care” to control chronic periodontitis and to maintain periodontal health. Pain or discomfort is often associated with non-surgical plaque removal. Ibuprofen arginine, because of its rapid onset of action and long duration, its favourable safety profile and the possibility of easy oral administration shortly before a dental procedure, is a promising agent to achieve pain control during and after periodontal scaling and root planing (SRP). For patients with mild to moderate chronic adult periodontitis treated in a general dental practice, a single dose of 800 mg ibuprofen arginine taken 30 minutes before treatment proved to be an effective and safe medication for maximising comfort during treatment, reducing average and maximum pain levels during SRP.
Gingival irritation and tooth sensitivity are the most common side effects of vital tooth bleaching.
Patients who experienced these side effects terminated treatment due to discomfort. A single dose of ibuprofen 600 mg administered preoperatively, decreases tooth sensitivity associated with in-office bleaching.
Finally, considering the low risk to develop gastrointestinal (214–216), liver (217–220) or cardiovascular toxicity (237,238), ibuprofen represent a good choice in the relief of dental pain and postoperative dental pain in children and adults. Moreover, considering also the optimal pharmacokinetic profile, ibuprofen may be also used in patients with poly-drug treatment due its low risk to develop drug-drug interaction.
Figure 5.
Faster onset of analgesia with ibuprofen granules than with ibuprofen tablets in patients with pain following dental surgery [50].
Table 2.
Evaluation of different doses of soluble ibuprofen tablets in postoperative dental pain. Distribution of the overall assessment scores for the various doses of ibuprofen and placebo as evaluated on a five-point global scale.
| Very Poor | Poor | Satisfactory | Good | Very Good | Not recorded | |
|---|---|---|---|---|---|---|
| Placebo | 2 | 5 | 10 | 3 | 0 | 1 |
| Ibuprofen tablets 200mg* | 0 | 6 | 6 | 5 | 3 | 2 |
| Ibuprofen tablets 400mg* | 0 | 6 | 6 | 5 | 3 | 2 |
| Ibuprofen tablets 600mg* | 0 | 0 | 4 | 9 | 7 | 1 |
| Soluble ibuprofen 200 mg* | 0 | 4 | 6 | 6 | 5 | 0 |
| Soluble ibuprofen 200 mg* | 0 | 1 | 3 | 8 | 6 | 3 |
| Soluble ibuprofen 200 mg* | 0 | 2 | 6 | 8 | 5 | 9 |
Significant differences (p<0.05) in the distribution of scores from placebo treatment.
Acknowledgements
Abbott provided compensation to the authors and provided funding to CIC Edizioni Internazionali for medical writing assistance and editorial support in the development of this review article. The authors wish to acknowledge CIC Edizioni Internazionali, for supporting and editorial assistance. Abbott had the opportunity to review and comment on the publication; however, all decisions regarding content were made by the authors.
References
- 1.Matthew JD. Pain management for dentists. TFDA. 2008;8:33–37. [PubMed] [Google Scholar]
- 2.Donaldson M, Goodchild JH. Appropriate analgesic prescribing for the general dentist. Gen Dent. 2010;58(4):291–7. [PubMed] [Google Scholar]
- 3.Menhinick KA, Gutmann JL, Regan JD, Taylor SE, Buschang PH. The efficacy of pain control following nonsurgical root canal treatment using ibuprofen or a combination of ibuprofen and acetaminophen in a randomized, double-blind, placebo-controlled study. Int Endod J. 2004;37(8):531–41. doi: 10.1111/j.1365-2591.2004.00836.x. [DOI] [PubMed] [Google Scholar]
- 4.Johnsen DC, Harshbarger J, Rymer HD. Quantitative assessment of neural development in human premolars. AnatRec. 1983;205:421–429. doi: 10.1002/ar.1092050407. [DOI] [PubMed] [Google Scholar]
- 5.Malmberg AB, Yaksh TL. Hyperalgesia mediated by spinal glutamate or substance P receptor blocked by spinal cyclooxygenase inhibition. Science. 1992;257:1276–1279. doi: 10.1126/science.1381521. [DOI] [PubMed] [Google Scholar]
- 6.Dubner R, Bennett GJ. Spinal and trigeminal mechanisms of nociception. Annu Rev Neurosci. 1983;6:381–418. doi: 10.1146/annurev.ne.06.030183.002121. [DOI] [PubMed] [Google Scholar]
- 7.Kalyvas DG, Tarenidou M. Influence of nonsteroidal anti-inflammatory drugs on osseointegration. J Oral Sci. 2008;50:239–246. doi: 10.2334/josnusd.50.239. [DOI] [PubMed] [Google Scholar]
- 8.Celotti F, Laufer S. Anti-inflammatory drugs: new multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol Res. 2001;43:429–436. doi: 10.1006/phrs.2000.0784. [DOI] [PubMed] [Google Scholar]
- 9.Ferreira SH. Peripheral analgesic sites of action of anti-inflammatory drugs. Int J Clin Pract Suppl. 2002;(128):2–10. [PubMed] [Google Scholar]
- 10.Ong CK, Seymour RA. An evidence-based update of the use of analgesics in dentistry. Periodontol 2000. 2008;46:143–164. doi: 10.1111/j.1600-0757.2008.00225.x. [DOI] [PubMed] [Google Scholar]
- 11.Battistini B, Botting R, Bakhle YS. COX-1 and COX-2: toward the development of more selective NSAIDs. Drugs News Perspect. 1994;7:501–512. [Google Scholar]
- 12.Cryer B, Feldman M. Cyclooxygenase-1 and cyclooxygenase-2 selectivity of widely used nonsteroidal anti-inflammatory drugs. Am J Med. 1998;104:413–421. doi: 10.1016/s0002-9343(98)00091-6. [DOI] [PubMed] [Google Scholar]
- 13.Graul A, Martel AM, Castaner J. Celecoxib: anti-inflammatory cyclooxygenase-2 inhibitor. Drugs of the Future. 1997;22:711–714. [Google Scholar]
- 14.Clissold SP. Paracetamol and phenacetin. Drugs. 1986;32:46–59. doi: 10.2165/00003495-198600324-00005. [DOI] [PubMed] [Google Scholar]
- 15.Vane JR, Botting RM. Anti-inflammatory drugs and their mechanism of action. Inflamm Res. 1998;47:S78–S87. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 16.Davies NM, Good RL, Roupe KA, Yáñez JA. Cyclooxygenase-3: axiom, dogma, anomaly, enigma or splice error? – Not as easy as 1, 2, 3. JPharm Pharm Sci. 2004;7:217–226. [PubMed] [Google Scholar]
- 17.Serhan CN, Maddox JF, Petasis NA, et al. Design of lipoxin A4 stable analogs that block transmigration and adhesion of human neutrophils. Biochemistry. 1995;34:14609–14615. doi: 10.1021/bi00044a041. [DOI] [PubMed] [Google Scholar]
- 18.Claria J, Lee MH, Serhan CN. Aspirin-triggered lipoxins (15-epi-LX) are generated by the human lung adenocarcinoma cell line (A549)-neutrophil interactions and are potent inhibitors of cell proliferation. Mol Med. 1996;2:583–596. [PMC free article] [PubMed] [Google Scholar]
- 19.Serhan CN. Novel omega–3-derived local mediators in anti-inflammation and resolution. Pharmacol Ther. 2005;105:7–21. doi: 10.1016/j.pharmthera.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Adams SS. The propionic acids: a personal perspective. J Clin Pharmacol. 1992;32:317–323. doi: 10.1002/j.1552-4604.1992.tb03842.x. [DOI] [PubMed] [Google Scholar]
- 21.Seymour RA, Walton JG. Pain control after third molar surgery. Int J Oral Surg. 1984;13:457–485. doi: 10.1016/s0300-9785(84)80017-4. [DOI] [PubMed] [Google Scholar]
- 22.Frame JW, Evans CR, Flaum GR, Langford R, Rout PG. A comparison of ibuprofen and dihydrocodeine in relieving pain following wisdom teeth removal. Br Dent J. 1989;166:121–124. doi: 10.1038/sj.bdj.4806746. [DOI] [PubMed] [Google Scholar]
- 23.Hill CM, Carroll MJ, Giles AD, Pickvance N. Ibuprofen given pre-and post-operatively for the relief of pain. Int J Oral Maxillofac Surg. 1987;16:420–424. doi: 10.1016/s0901-5027(87)80078-4. [DOI] [PubMed] [Google Scholar]
- 24.Jain AK, Ryan JR, McMahon FG, Kuebel JO, Walters PJ, Noveck C. Analgesic efficacy of low-dose ibuprofen in dental extraction pain. Pharmacotherapy. 1986;6:318–322. doi: 10.1002/j.1875-9114.1986.tb03494.x. [DOI] [PubMed] [Google Scholar]
- 25.Adams SS, Bresloff P, Mason CG. Pharmacological differences between the optical isomers of ibuprofen; evidence for metabolic inversion of the (−)-isomer. J Pharm Pharmacol. 1976;28:256–257. doi: 10.1111/j.2042-7158.1976.tb04144.x. [DOI] [PubMed] [Google Scholar]
- 26.Gaut ZN, Baruth H, Randall LO, Ashley C, Paulsrud JR. Stereoisomeric relationships among anti-inflammatory activity, inhibition of platelet aggregation, and inhibition of prostaglandin synthetase. Prostaglandins. 1975;10:59–66. doi: 10.1016/0090-6980(75)90093-3. [DOI] [PubMed] [Google Scholar]
- 27.Evans AM, Nation RL, Sansom LN, Bochner F, Somogyi AA. Effect of racemic ibuprofen dose on the magnitude and duration of platelet cycleoxygenase inhibition: relationship between inhibition of thromboxane production and the plasma unbound concentration of S(+)-ibuprofen. Br J Clin Pharmacol. 1991;31:131–38. doi: 10.1111/j.1365-2125.1991.tb05500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rainsford KD. Discovery, mechanisms of action and safety of ibuprofen. Int J Clin Pract Suppl. 2003:3–8. [PubMed] [Google Scholar]
- 29.Jamali F, Mehvar R, Russell AS, Sattari S, Yakimets WW, Koo J. Human pharmacokinetics of ibuprofen enantiomers following different doses and formulations: intestinal chiral inversion. J Pharm Sci. 1992;81:221–225. doi: 10.1002/jps.2600810306. [DOI] [PubMed] [Google Scholar]
- 30.Walson PD, Galletta G, Braden NJ, Alexander L. Ibuprofen, acetaminophen, and placebo treatment of febrile children. Clin Pharmacol Ther. 1989;46:9–17. doi: 10.1038/clpt.1989.100. [DOI] [PubMed] [Google Scholar]
- 31.Mäkelä A-L, Lempiänen M, Yrjänä T. Ibuprofen in the treatment of juvenile rheumatoid arthritis: Metabolism and concentrations in synovial fluid. Brit J Clin Pract. 1980;6:23–27. [Google Scholar]
- 32.Ferreira SH, Moncada S, Vane JR. Prostaglandins and the mechanism of analgesia produced by aspirin-like drugs. Br J Pharmacol. 1973;49:86–97. doi: 10.1111/j.1476-5381.1973.tb08270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferreira SH. Local control of inflammatory pain. Agents Actions. 1981;11:636–638. doi: 10.1007/BF01978773. [DOI] [PubMed] [Google Scholar]
- 34.Nielsen JC, Bjerring P, Arendt-Nielsen L, Petterson KJ. A double-blind, placebo controlled, cross-over comparison of the analgesic effect of ibuprofen 400 mg and 800 mg on laser-induced pain. Br J Clin Pharmacol. 1990;30:711–715. doi: 10.1111/j.1365-2125.1990.tb03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dubinsky B, Schupsky JJ. Mechanism of action of suprofen, a new peripheral analgesic, as demonstrated by its effects on several nociceptive mediators. Prostaglandins. 1984;28:241–252. doi: 10.1016/0090-6980(84)90060-1. [DOI] [PubMed] [Google Scholar]
- 36.Fowler CJ, Stenström A, Tiger G. Ibuprofen inhibits the metabolism of the endogenous cannabimimetic agent anandamide. Pharmacol Toxicol. 1997;80:103–107. doi: 10.1111/j.1600-0773.1997.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 37.Fowler CJ, Janson U, Johnson RM, Wahlstrom G, Stenstrom A, Norstrom K, Tiger G. Inhibition of anandamide hydrolysis by the enantiomers of ibuprofen, ketorolac, and flurbiprofen. Arc Biochem Biophys. 1999;362:191–6. doi: 10.1006/abbi.1998.1025. [DOI] [PubMed] [Google Scholar]
- 38.Guindon J, De Léan A, Beaulieu P. Local interactions between anandamide, an endocannabinoid, and ibuprofen, a nonsteroidal anti-inflammatory drug, in acute and inflammatory pain. Pain. 2006;121:85–93. doi: 10.1016/j.pain.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 39.Guindon J, Beaulieu P. The Role of the Endogenous Cannabinoid System in Peripheral Analgesia. Curr Mol Pharmacol. 2009;2:134–139. doi: 10.2174/1874467210902010134. [DOI] [PubMed] [Google Scholar]
- 40.Hargreaves KM, Dionne RA, Mueller GP, Goldstein DS, Dubner R. Naloxone, fentanyl, and diazepam modify plasma beta-endorphinlevels during surgery. Clin Pharmacol Ther. 1986;40:165–171. doi: 10.1038/clpt.1986.159. [DOI] [PubMed] [Google Scholar]
- 41.Dionne RA, McCullagh L. The S(+) isomer of ibuprofen-suppresses plasma beta-endorphin coincident with analgesia inhumans. Clin Pharmacol Ther. 1998;63:694–701. doi: 10.1016/S0009-9236(98)90094-7. [DOI] [PubMed] [Google Scholar]
- 42.Lokken P, Olsen I, Bruaset I, Norman-Pedersen K. Bilateralsurgical removal of impacted third molar teeth as a model fordrug evaluation: a test with ibuprofen. Eur J Clin Pharmacol. 1975;8:209–216. doi: 10.1007/BF00567117. [DOI] [PubMed] [Google Scholar]
- 43.Troullos ES, Hargreaves KM, Butler DP, Dionne RA. Comparison of non-steroidal anti-inflammatory drugs, ibuprofenand flurbiprofen, with methylprednisolone and placebofor acute pain, swelling and trismus. J Oral Maxillofac Surg. 1990;48:945–952. doi: 10.1016/0278-2391(90)90007-o. [DOI] [PubMed] [Google Scholar]
- 44.Schultze-Mosgau S, Schmelzeisen R, Frolich JC, Schmele H. Use of ibuprofen and methylprednisolone for the preventionof pain and swelling after removal of impacted third molars. J Oral Maxillofac Surg. 1995;53:2–7. doi: 10.1016/0278-2391(95)90486-7. [DOI] [PubMed] [Google Scholar]
- 45.Davies NM. Clinical pharmacokinetics of ibuprofen. The first 30 years. Clin Pharmacokinet. 1998;34:101–154. doi: 10.2165/00003088-199834020-00002. [DOI] [PubMed] [Google Scholar]
- 46.Forrest JAH, Clements JA, Prescott LF. Clinical pharmacokinetics of paracetamol. Clin Pharmacokinet. 1982;7:93–107. doi: 10.2165/00003088-198207020-00001. [DOI] [PubMed] [Google Scholar]
- 47.Lim JH, Cochetto DM, Duggan DE. Protein binding as a primary determinant of the clinical pharmacokinetic properties of non-steroidal anti-inflammatory drugs. Clin Pharmacokinet. 1987;12:402–432. doi: 10.2165/00003088-198712060-00002. [DOI] [PubMed] [Google Scholar]
- 48.Woodhouse KW, Wynne H. The pharmacokinetics of non-steroidal anti-inflammatory drugs in the elderly. Clin Pharmacokinet. 1987;12:111–122. doi: 10.2165/00003088-198712020-00002. [DOI] [PubMed] [Google Scholar]
- 49.Seymour RA, Hawkesford JE, Weldon M, Brewster D. An evaluation of different ibuprofen preparations in the control of postoperative pain after third molar surgery. Br J Clin Pharmacol. 1991;31:83–87. doi: 10.1111/j.1365-2125.1991.tb03861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sharma NK, Kindelan JD, Hutchinson D, Lancaster L. A study to compare ibuprofen effervescent granules with ibuprofen tablets in the treatment of acute dental pain. Prim Den Care. 1994;1:5–8. [PubMed] [Google Scholar]
- 51.Li G, Treiber G, Maier K, Walker S, Klotz U. Disposition of ibuprofen in patients with liver cirrhosis. Stereochemical considerations. Clin Pharmacokinet. 1993;25:154–163. doi: 10.2165/00003088-199325020-00008. [DOI] [PubMed] [Google Scholar]
- 52.Albert KS, Gernaat CM. Pharmacokinetics of ibuprofen. Am J Med. 1984;77:40–46. doi: 10.1016/s0002-9343(84)80017-0. [DOI] [PubMed] [Google Scholar]
- 53.Kepp DR, Sidelmann UG, Hansen SH. Isolation and characterization of major phase I and II metabolites of ibuprofen. Pharm Res. 1997;14:676–680. doi: 10.1023/a:1012125700497. [DOI] [PubMed] [Google Scholar]
- 54.Shirley MA, Guan X, Kaiser DG, Halstead GW, Baillie TA. Taurine conjugation of ibuprofen in humans and in rat liver in vitro. Relationship to metabolic chiral inversion. J Pharmacol Exp Ther. 1994;269:1166–1175. [PubMed] [Google Scholar]
- 55.Schneider HT, Nuernberg B, Dietzel K, Brune K. Biliary elimination on non-steroidal anti-inflammatory drugs in patients. Br J Clin Pharmacol. 1990;29:127–131. doi: 10.1111/j.1365-2125.1990.tb03613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strassburg CP, Strassburg A, Kneip S, et al. Developmental aspects of human hepatic drug glucuronidation in young adults and children. Gut. 2002;50:259–265. doi: 10.1136/gut.50.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laska EM, Sunshine A, Marrero I, Olson N, Siegel C, McCormick N. The correlation between blood levels of ibuprofen and clinical analgesic response. Clin Pharmacol Ther. 1986;40:1–7. doi: 10.1038/clpt.1986.129. [DOI] [PubMed] [Google Scholar]
- 58.Benvenuti C, Cancellieri V, Gambaro V, Lodi F, Marozzi E, Scaroni C. Pharmacokinetics of two new oral formulations of ibuprofen. Int J Clin Pharmacol Ther Toxicol. 1986;24:308–312. [PubMed] [Google Scholar]
- 59.Walton RE, Reader AF. Local anesthesia. In: Walton RE, Torabinejad M, editors. Principles and practice of endodontics. 3rd ed. Philadelphia: W.B. Saunders Company; 2002. pp. 99–117. [Google Scholar]
- 60.Wallace JA, Michanowicz AE, Mundell RD, Wilson EG. A pilot study of the clinical problem of regionally anesthetizing the pulp of an acutely inflamed mandibular molar. Oral Surg Oral Med Oral Pathol. 1985;59:517–521. doi: 10.1016/0030-4220(85)90095-7. [DOI] [PubMed] [Google Scholar]
- 61.Malamed SF. Handbook of Local Anesthesia. 4th ed. St Louis: Mosby; 1997. [Google Scholar]
- 62.Gutmann JL, Dumsha T. Problem solving in endodontics. In: Gutmann JL, Dumsha TC, Lovdahl PE, Hovland EG, editors. Problems in Managing Endodontic emergencies. St Louis: C.V. Mosby; 1997. [Google Scholar]
- 63.Hargreaves KM, Keiser K. Local anesthetic failure in endodontics: mechanisms and management. Endodontic Topics. 2002;1:26–39. [Google Scholar]
- 64.Rood JP, Pateromichelakis S. Inflammation and peripheral nerve sensitisation. Br J Oral Surg. 1981;19:67–72. doi: 10.1016/0007-117x(81)90023-8. [DOI] [PubMed] [Google Scholar]
- 65.Cohen S, Liewehr F. Diagnostic procedures. In: Cohen S, Burns RC, editors. Pathways of the pulp. 8th edition. St Louis (MO): Mosby; 2002. pp. 3–30. [Google Scholar]
- 66.Ingle JI, Walton RE, Malamed SF, et al. Preparation for endodontic treatment. In: Ingle JI, Bakland LK, editors. Endodontics. 5th ed. Hamilton: BC Decker Inc; 2002. pp. 357–404. [Google Scholar]
- 67.Cohen HP, Cha BY, Spångberg LS. Endodontic anesthesia in mandibular molars: a clinical study. J Endod. 1993;19:370–373. doi: 10.1016/S0099-2399(06)81366-X. [DOI] [PubMed] [Google Scholar]
- 68.Nusstein J, Reader A, Nist R, Beck M, Meyers WJ. Anesthetic efficacy of the supplemental intraosseous injection of 2% lidocaine with 1:100,000 epinephrine in irreversible pulpitis. J Endod. 1998;24:487–491. doi: 10.1016/S0099-2399(98)80053-8. [DOI] [PubMed] [Google Scholar]
- 69.Reisman D, Reader A, Nist R, Beck M, Weaver J. Anesthetic efficacy of the supplemental intraosseous injection of 3% mepivacaine in irreversible pulpitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:676–682. doi: 10.1016/s1079-2104(97)90372-3. [DOI] [PubMed] [Google Scholar]
- 70.Nakanishi T, Matsuo T, Ebisu S. Quantitative analysis of immunoglobulins and inflammatory factors in human pulpal blood from exposed pulps. J Endod. 1995;21:131–136. doi: 10.1016/s0099-2399(06)80438-3. [DOI] [PubMed] [Google Scholar]
- 71.Hargreaves KM, Keiser K, Byrne BE. Analgesics in endodontics. In: Cohen S, Hargreaves KM, editors. Pathways of the Pulp. Ninth ed. St Louis: Mosby Elsevier; 2006. pp. 668–90. [Google Scholar]
- 72.Henry MA, Hargreaves KM. Peripheral mechanisms of odontogenic pain. Dent Clin North Am. 2007;51:19–44. doi: 10.1016/j.cden.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 73.Wassef M, Pelage JP, Velzenberger E, et al. Anti-inflammatory effect of ibuprofen-loaded embolization beads in sheep uterus. J Biomed Mater Res B Appl Biomater. 2008;86:63–73. doi: 10.1002/jbm.b.30988. [DOI] [PubMed] [Google Scholar]
- 74.Gould HJ, 3rd, England JD, Soignier RD, et al. Ibuprofen blocks changes in Nav 1.7 and 1.8 sodium channels associated with complete Freund’s adjuvant-induced inflammation in rat. J Pain. 2004;5:270–280. doi: 10.1016/j.jpain.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 75.Gibbs JL, Hargreaves KM. Mechanisms of odontogenic and non-odontogenic pain. In: Ingle JI, Bakland LK, Baumgartner JC, editors. Ingle's Endodontics 6. BC Decker Inc; 2008. pp. 376–391. [Google Scholar]
- 76.Ianiro SR, Jeansonne BG, McNeal SF, Eleazer PD. The effect of preoperative acetaminophen or a combination of acetaminophen and Ibuprofen on the success of inferior alveolar nerve block for teeth with irreversible pulpitis. J Endod. 2007;33:11–14. doi: 10.1016/j.joen.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 77.Modaresi J, Dianat O, Mozayeni MA. The efficacy comparison of ibuprofen, acetaminophen-codeine, and placebo premedication therapy on the depth of anesthesia during treatment of inflamed teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;102:399–403. doi: 10.1016/j.tripleo.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 78.Seymour RA, Ward-Booth P, Kelly PJ. Evaluation of different doses of soluble ibuprofen and ibuprofen tablets in postoperative dental pain. Br J Oral Maxillofac Surg. 1996;34:110–114. doi: 10.1016/s0266-4356(96)90147-3. [DOI] [PubMed] [Google Scholar]
- 79.Parirokh M, Ashouri R, Rekabi AR, et al. The effect of premedication with ibuprofen and indomethacin on the success of inferior alveolar nerve block for teeth with irreversible pulpitis. J Endod. 2010;36:1450–1454. doi: 10.1016/j.joen.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 80. [Accessed November 1, 2010]. http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=meds&log$=,I.w.p.N.C.f.B.I.w.A.a.and_drug_bottom_one&part=a681027.
- 81.Hargreaves KM, Hutter JW. Endodontic pharmacology: pain management strategies. In: Cohen S, Burns R, editors. Pathways of the Pulp. St Louis: Mosby; 2002. pp. 665–682. [Google Scholar]
- 82.Fouad AF. Molecular mediators of pulpal inflammation. In: Hargreaves KM, Goodis HE, editors. Seltzer and Bender's dental pulp. Chicago: Quintessence Publishing Co.; 2002. pp. 247–279. [Google Scholar]
- 83.Hargreaves KM, Seltzer S. Pharmacologic control of dental pain. In: Hargreaves KM, Goodis HE, editors. Seltzer’s& Bender’s Dental Pulp Carol Stream. IL: Quintessence Publishing Co; 2002. pp. 205–225. [Google Scholar]
- 84.Lipton J, Ship JA, Larach-Robinson D. Estimated prevalence and distribution of reported of orofacial pain in the United States. J Am Dent Assoc. 1993;124:115–121. doi: 10.14219/jada.archive.1993.0200. [DOI] [PubMed] [Google Scholar]
- 85.Wideman G, Keffer M, Morris E, Doyle RT, Jr, Jiang JG, Beaver WT. Analgesic efficacy of a combination of hydrocodone with Ibuprofen in postoperative pain. Clin Pharmacol Ther. 1999;65:66–76. doi: 10.1016/S0009-9236(99)70123-2. [DOI] [PubMed] [Google Scholar]
- 86.Mickel AK, Wright AP, Chogle S, Jones JJ, Kantorovich I, Curd F. An analysis of current analgesic preferences for endodontic pain management. J Endod. 2006;32:1146–1154. doi: 10.1016/j.joen.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 87.Cooper SA. The relative efficacy of ibuprofen in dental pain. Compend Contin Educ Dent. 1986;7:578–588. [PubMed] [Google Scholar]
- 88.Sutherland S, Matthews DC. Emergency management of acute apical periodontitis in the permanent dentition: a systematic review of the literature. J Can Dent Assoc. 2003;69:160. [PubMed] [Google Scholar]
- 89.Haas DA. Local and systemic therapeutics for the control of endodontic pain. Alpha Omegan. 1997;90:73–76. [PubMed] [Google Scholar]
- 90.Krasner P, Jackson E. Management of posttreatment endodontic pain with oral dexa-methasone: a double-blind study. Oral Surg Oral Med Oral Pathol. 1986;62:187–190. doi: 10.1016/0030-4220(86)90044-7. [DOI] [PubMed] [Google Scholar]
- 91.Chong BS, Pitt Ford TR. Postoperative pain after root-end resection and filling. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:762–766. doi: 10.1016/j.tripleo.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 92.Kvist T, Reit C. Postoperative discomfort associated with surgical and nonsurgical endodontic retreatment. Endod Dent Traumatol. 2000;16:71–74. doi: 10.1034/j.1600-9657.2000.016002071.x. [DOI] [PubMed] [Google Scholar]
- 93.Houck V, Reader A, Beck M, Nist R, Weaver J. Effect of trephination on postoperative pain and swelling in symptomatic necrotic teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:507–513. doi: 10.1067/moe.2000.108960. [DOI] [PubMed] [Google Scholar]
- 94.Gopikrishna V, Parameswaran A. Effectiveness of prophylactic use of rofecoxib in comparison with Ibuprofen on postendodontic pain. J Endod. 2003;29:62–64. doi: 10.1097/00004770-200301000-00017. [DOI] [PubMed] [Google Scholar]
- 95.Litkowski LJ, Christensen SE, Adamson DN, Van Dyke T, Han SH, Newman KB. Analgesic efficacy and tolerability of oxycodone 5 mg/ibuprofen 400 mg compared with those of oxycodone 5 mg/acetaminophen 325 mg and hydrocodone 7.5 mg/acetaminophen 500 mg in patients with moderate to severe postoperative pain: a randomized, double-blind, placebo-controlled, single-dose, parallel-group study in a dental pain model. Clin Ther. 2005;27:418–429. doi: 10.1016/j.clinthera.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 96.Keenan JV, Farman AG, Fedorowicz Z, Newton JT. A Cochrane systematic review finds no evidence to support the use of antibiotics for pain relief in irreversible pulpitis. J Endod. 2006;32:87–92. doi: 10.1016/j.joen.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 97.Hitchcock M, Ogg TW. Anaesthesia for day-case surgery. Br J Hosp Med. 1995;54:202–206. [PubMed] [Google Scholar]
- 98.Cooper SA. Models for clinical assessment of oral analgesics. Am J Med. 1983;75(Suppl 5A):24–29. doi: 10.1016/0002-9343(83)90229-2. [DOI] [PubMed] [Google Scholar]
- 99.Cooper SA, Schachtel BP, Goldman E, Gelb S, Cohn P. Ibuprofen and acetaminophen in the relief of acute pain: a randomized, double-blind, placebo-controlled study. J Clin Pharmacol. 1989;29:1026–1030. doi: 10.1002/j.1552-4604.1989.tb03273.x. [DOI] [PubMed] [Google Scholar]
- 100.Seymour RA, Frame J, Negus TW, Hawkesford JT, Marsden J, Matthew IR. The comparative efficacy of aceclofenac and ibuprofen in post-operative pain after third molar surgery. Br J Oral Maxillofac Surg. 1998;36:375–379. doi: 10.1016/s0266-4356(98)90650-7. [DOI] [PubMed] [Google Scholar]
- 101.Cooper SA, Beaver WT. A model to evaluate mild analgesics in oral surgery outpatients. Clin Pharmacol Ther. 1976;20:241–250. doi: 10.1002/cpt1976202241. [DOI] [PubMed] [Google Scholar]
- 102.Dionne RA, Campbell RA, Cooper SA, Hall DL, Buckingham B. Suppression of postoperative pain by preoperative administration of ibuprofen in comparison to placebo, acetaminophen, and acetaminophen plus codeine. J Clin Pharmacol. 1983;23:37–43. doi: 10.1002/j.1552-4604.1983.tb02702.x. [DOI] [PubMed] [Google Scholar]
- 103.Dionne RA, Cooper SA. Evaluation of preoperative ibuprofen for postoperative pain after removal of third molars. Oral Surg Oral Med Oral Pathol. 1978;45:851–856. doi: 10.1016/s0030-4220(78)80004-8. [DOI] [PubMed] [Google Scholar]
- 104.Barden J, Edwards JE, McQuay HJ, Andrew Moore R. Pain and analgesic response after third molar extraction and other postsurgical pain. Pain. 2004;107:86–90. doi: 10.1016/j.pain.2003.09.021. [DOI] [PubMed] [Google Scholar]
- 105.Ahlstrom U, Bakshi R, Nilsson P, Wåhlander L. The analgesic efficacy of diclofenac dispersible and ibuprofen in postoperative pain after dental extraction. Eur J Clin Pharmacol. 1993;44:587–588. doi: 10.1007/BF02440865. [DOI] [PubMed] [Google Scholar]
- 106.Dionne R. Relative efficacy of selective COX-2 inhibitors compared with over-the-counter ibuprofen. Int J Clin Pract Suppl. 2003;(135):18–22. [PubMed] [Google Scholar]
- 107.Campbell WI, Kendrick R, Patterson C. Intravenous diclofenac sodium does its administration before operation suppress postoperative pain? Anaesthesia. 1990;45:763–766. doi: 10.1111/j.1365-2044.1990.tb14450.x. [DOI] [PubMed] [Google Scholar]
- 108.Richmond CE, Bromley LM, Woolf CJ. Preoperative morphine preempts postoperative pain. Lancet. 1993;342:73–75. doi: 10.1016/0140-6736(93)91284-s. [DOI] [PubMed] [Google Scholar]
- 109.Collis R, Brandner B, Bromley LM, Woolf CJ. Is there any clinical advantage of increasing the pre-emptive dose of morphine or combining pre-incisional with postoperative morphine administration? Br J Anaesth. 1995;74:396–399. doi: 10.1093/bja/74.4.396. [DOI] [PubMed] [Google Scholar]
- 110.Yoon DM, Ahn EY, Lee YW. The effect of pre-emptive analgesia with intravenous morphine for post-operative pain relief. Eighth World Congress on Pain, International Association for the Study of Pain; Seattle. Seattle: IASP Press; 1996. [Google Scholar]
- 111.Kissin I. Preemptive analgesia: problems with assessment of clinical significance. Methods Mol Biol. 2010;617:475–482. doi: 10.1007/978-1-60327-323-7_34. [DOI] [PubMed] [Google Scholar]
- 112.Kissin I. Preemptive analgesia. Anesthesiology. 2000;93:1138–1143. doi: 10.1097/00000542-200010000-00040. [DOI] [PubMed] [Google Scholar]
- 113.Schou S, Nielsen H, Nattestad A, et al. Analgesic dose-response relationship of ibuprofen 50, 100, 200 and 400mg after surgical removal of third molars: a single dose, randomised, placebo-controlled double-blind study of 304 patients. Clin Pharmacol. 1998;385:447–454. doi: 10.1002/j.1552-4604.1998.tb04452.x. [DOI] [PubMed] [Google Scholar]
- 114.Jones K, Seymour RA, Hawkesford JE. Are the pharmacokinetics of ibuprofen important determinants for the drug’s efficacy in postoperative pain after third molar surgery? Br J Oral Maxillofac Surg. 1997;35:173–176. doi: 10.1016/s0266-4356(97)90558-1. [DOI] [PubMed] [Google Scholar]
- 115.Joshi A, Snowdon AT, Rood JP, Worthington HV. Pain control after routine dento-alveolar day surgery: a patient satisfaction survey. Br Dent J. 200;189:439–442. doi: 10.1038/sj.bdj.4800794. [DOI] [PubMed] [Google Scholar]
- 116.Cooper SA, Precheur H, Rauch D, Rosenheck A, Ladou M, Engel J. Evaluation of oxycodone and acetaminophen in the treatment of post-operative dental pain. Oral Surg Oral Med Oral Pathol. 1980;50:496–501. doi: 10.1016/0030-4220(80)90430-2. [DOI] [PubMed] [Google Scholar]
- 117.Desjardins PJ, Shu VS, Recker DP, Verburg KM, Woolf CJ. A single preoperative oral dose of valdecoxib, a new cyclooxygenase-2 specific inhibitor, relieves post-oral surgery or bunionectomy pain. Anesthesiology. 2002;97:565–573. doi: 10.1097/00000542-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 118.Woolf CJ, Chong MS. Pre-emptive analgesia – treating post-operative pain by preventing the establishment of central sensitization. Anesth Analg. 1993;77:362–379. doi: 10.1213/00000539-199377020-00026. [DOI] [PubMed] [Google Scholar]
- 119.Dahl JB, Kehlet H. The value of pre-emptive analgesia in the treatment of post-operative pain. Br J Anaesth. 1993;70:434–439. doi: 10.1093/bja/70.4.434. [DOI] [PubMed] [Google Scholar]
- 120.McQuay H. Pre-emptive analgesia: a systematic review of clinical studies. Ann Med. 1995;27:249–256. doi: 10.3109/07853899509031967. [DOI] [PubMed] [Google Scholar]
- 121.Møiniche S, Kehlet H, Dahl JB. A qualitative and quantitative systematic review of preemptive analgesia for postoperative pain relief: the role of timing of analgesia. Anesthesiology. 2002;96:725–741. doi: 10.1097/00000542-200203000-00032. [DOI] [PubMed] [Google Scholar]
- 122.Sisk AL, Mosley RO, Martin RP. Comparison of pre-operative and post-operative diflunisal for suppression of post-operative pain. J Oral Maxillofac Surg. 1989;47:464–468. doi: 10.1016/0278-2391(89)90278-4. [DOI] [PubMed] [Google Scholar]
- 123.Sisk AL, Grover BJ. A comparison of preoperative and postoperative naproxen sodium for suppression of postoperative pain. J Oral Maxillofac Surg. 1990;48:674–678. doi: 10.1016/0278-2391(90)90048-7. [DOI] [PubMed] [Google Scholar]
- 124.Bridgman JB, Gillgrass TG, Zacharias M. The absence of any preemptive analgesic effect for non-steroidal anti-inflammatory drugs. Br J Oral Maxillofac Surg. 1996;34:428–431. doi: 10.1016/s0266-4356(96)90101-1. [DOI] [PubMed] [Google Scholar]
- 125.Gustafsson I, Nystrom E, Quiding H. Effect of preoperative paracetamol on pain after oral surgery. Eur J Clin Pharmacol. 1983;24:63–65. doi: 10.1007/BF00613928. [DOI] [PubMed] [Google Scholar]
- 126.Dupuis R, Lemay H, Bushnell MC, Duncan GH. Preoperative flurbiprofen in oral surgery: a method of choice in controlling postoperative pain. Pharmacotherapy. 1988;8:193–200. doi: 10.1002/j.1875-9114.1988.tb04074.x. [DOI] [PubMed] [Google Scholar]
- 127.Joshi A, Parara E, Macfarlane TV. A double-blind randomised controlled clinical trial of the effect of preoperative ibuprofen, diclofenac, paracetamol with codeine and placebo tablets for relief of postoperative pain after removal of impacted third molars. Br J Oral Maxillofac Surg. 2004;42:299–306. doi: 10.1016/j.bjoms.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 128.Esteller-Martínez V, Paredes-García J, Valmaseda-Castellón E, Berini-Aytés L, Gay-Escoda C. Analgesic efficacy of diclofenac sodium versus ibuprofen following surgical extraction of impacted lower third molars. Med Oral Patol Oral Cir Bucal. 2004;9:448–453. 444–448. [PubMed] [Google Scholar]
- 129.Barden J, Edwards JE, McQuay HJ, Wiffen PJ, Moore RA. Relative efficacy of oral analgesics after third molar extraction. Br Dent J. 2004;197:407–11. doi: 10.1038/sj.bdj.4811721. [DOI] [PubMed] [Google Scholar]
- 130.Averbuch M, Katzper M. Severity of baseline pain and degree of analgesia in the third molar post-extraction dental pain model. Anesth Analg. 2003;97:163–167. doi: 10.1213/01.ane.0000063827.97392.5e. [DOI] [PubMed] [Google Scholar]
- 131.Zamiril B, Mousavizadeh K, Tajoddini M, Mohammadinezhad C, Aarabi AM. Comparison of Ibuprofen, Celecoxib and Tramadol in Relief of Pain after Extraction of Mandibular Third Molar Teeth. IR-CMJ. 2009;11(4):431–436. [Google Scholar]
- 132.Jamali F, Kun-Dober C. Pain-mediated altered absorpition and metabolism of ibuprofen: an explanation for decreased serum enantiomer concentration after dental surgery. Br J Clin Pharmacol. 1999;47:391–6. doi: 10.1046/j.1365-2125.1999.00902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Forbes JA, Kehm CJ, Grodin CD. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen-codeine combination in postoperative oral surgery pain. Pharmacotherapy. 1990;10:94S–105S. [PubMed] [Google Scholar]
- 134.Ram D, Peretz B. Administering local anaesthesia to paediatric dental patients - current status and prospects for the future. Int J Paediatr Dent. 2002;12:80–89. doi: 10.1046/j.1365-263x.2002.00343.x. [DOI] [PubMed] [Google Scholar]
- 135.Goddard JM, Pickup SE. Postoperative pain in children. Anaesthesia. 1996;51:588–590. doi: 10.1111/j.1365-2044.1996.tb12573.x. [DOI] [PubMed] [Google Scholar]
- 136.Primosch RE, Antony SJ, Courts FJ. The efficacy of preoperative analgesic administration for postoperative pain management of pediatric dental patients. Anesth Pain Control Dent. 1993;2:102–106. [PubMed] [Google Scholar]
- 137.Perrott DA, Goodenough B, Champion GD. Children's ratings of the intensity and unpleasantness of post-operative pain using facial expression scales. Eur J Pain. 2004;8:119–127. doi: 10.1016/S1090-3801(03)00087-9. [DOI] [PubMed] [Google Scholar]
- 138.Drendel AL, Gorelick MH, Weisman SJ, Lyon R, Brousseau DC, Kim MK. A randomized clinical trial of ibuprofen versus acetaminophen with codeine for acute pediatric arm fracture pain. Ann Emerg Med. 2009;54:553–560. doi: 10.1016/j.annemergmed.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 139.Sharaf AA. Evaluation of mandibular infiltration versus block anesthesia in pediatric dentistry. ASDC J Dent Child. 1997;64:276–281. [PubMed] [Google Scholar]
- 140.Pogrel MA, Bryan J, Regezi J. Nerve damage associated with inferior alveolar nerve blocks. J Am Dent Assoc. 1995;126:1150–1155. doi: 10.14219/jada.archive.1995.0336. [DOI] [PubMed] [Google Scholar]
- 141.Marinho RO. Abducent nerve palsy following dental local analgesia. Br Dent J. 1995;179:69–70. doi: 10.1038/sj.bdj.4808836. [DOI] [PubMed] [Google Scholar]
- 142.Gazal G, Bowman R, Worthington HV, Mackie IC. A double-blind randomized controlled trial investigating the effectiveness of topical bupivacaine in reducing distress in children following extractions under general anaesthesia. Int J Paediatr Dent. 2004;14:425–431. doi: 10.1111/j.1365-263X.2004.00587.x. [DOI] [PubMed] [Google Scholar]
- 143.Primosch RE, Nichols DL, Courts FJ. Comparison of preoperative ibuprofen, acetaminophen, and placebo administration on the parental report of postextraction pain in children. Pediatr Dent. 1995;17:187–191. [PubMed] [Google Scholar]
- 144.Pickering AE, Bridge HS, Nolan J, Stoddart PA. Double-blind, placebo controlled analgesic study of ibuprofen or rofecoxib in combination with paracetamol for tonsillectomy in children. Br J Anaesth. 2002;88:72–77. doi: 10.1093/bja/88.1.72. [DOI] [PubMed] [Google Scholar]
- 145.McGaw T, Raborn W, Grace M. Analgesics in pediatric dental surgery: relative efficacy of aluminum ibuprofen suspension and acetaminophen elixir. ASDC J Dent Child. 1987;54:106–109. [PubMed] [Google Scholar]
- 146.Gazal G, Mackie IC. A comparison of paracetamol, ibuprofen or their combination for pain relief following extractions in children under general anaesthesia: a randomized controlled trial. Int J Paediatr Dent. 2007;17:169–177. doi: 10.1111/j.1365-263X.2006.00806.x. [DOI] [PubMed] [Google Scholar]
- 147.Henry PJ. Oral implant restoration for enhanced oral function. Clin Exp Pharmacol Physiol. 2005;32:123–127. doi: 10.1111/j.1440-1681.2005.04140.x. [DOI] [PubMed] [Google Scholar]
- 148.Davis BK. Dental aesthetics and the aging patient. Facial Plast Surg. 2006;22:154–160. doi: 10.1055/s-2006-947722. [DOI] [PubMed] [Google Scholar]
- 149.Newman MG. The single-tooth implant as a standard of care. Int J Oral Maxillofac Implants. 1999;14:621–622. [PubMed] [Google Scholar]
- 150.Feine JS, Carlsson GE, Awad MA, et al. The McGill consensus statement on overdentures. Mandibular two-implant overdentures as first choice standard of care for edentulous patients. Gerodontology. 2002;19:3–4. [PubMed] [Google Scholar]
- 151.Engquist B, Astrand P, Dahlgren S, Engquist E, Feldmann H, Gröndahl K. Marginal bone reaction to oral implants: a prospective comparative study of Astra Tech and Branemark System implants. Clin Oral Implants Res. 2002;13:30–37. doi: 10.1034/j.1600-0501.2002.130103.x. [DOI] [PubMed] [Google Scholar]
- 152.Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399–410. doi: 10.1016/s0022-3913(83)80101-2. [DOI] [PubMed] [Google Scholar]
- 153.Jeffcoat MK, Williams RC, Reddy MS, English R, Goldhaber P. Flurbiprofen treatment of human periodontitis: effect on alveolar bone height and metabolism. J Periodontal Res. 1988;23:381–385. doi: 10.1111/j.1600-0765.1988.tb01617.x. [DOI] [PubMed] [Google Scholar]
- 154.Williams RC, Jeffcoat MK, Howell TH, et al. Altering the progression of human alveolar bone loss with the non-steroidal anti-inflammatory drug flurbiprofen. J Periodontol. 1989;60:485–490. doi: 10.1902/jop.1989.60.9.485. [DOI] [PubMed] [Google Scholar]
- 155.Jeffcoat MK, Page R, Reddy M, et al. Use of digital radiography to demonstrate the potential of naproxen as an adjunct in the treatment of rapidly progressive periodontitis. J Periodontal Res. 1991;26:415–421. doi: 10.1111/j.1600-0765.1991.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 156.Reddy MS, Palcanis KG, Barnett ML, Haigh S, Charles CH, Jeffcoat MK. Efficacy of meclofenamate sodium (Meclomen) in the treatment of rapidly progressive periodontitis. J Clin Periodontol. 1993;20:635–640. doi: 10.1111/j.1600-051x.1993.tb00708.x. [DOI] [PubMed] [Google Scholar]
- 157.Jeffcoat MK, Reddy MS, Wang IC, Meuninghoff LA, Farmer JB, Koth DL. The effect of systemic flurbiprofen on bone supporting dental implants. J Am Dent Assoc. 1995;126:305–311. doi: 10.14219/jada.archive.1995.0173. [DOI] [PubMed] [Google Scholar]
- 158.Gerstenfeld LC, Thiede M, Seibert K, et al. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res. 2003;21:670–675. doi: 10.1016/S0736-0266(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 159.Beck A, Krischak G, Sorg T, et al. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch Orthop Trauma Surg. 2003;123:327–332. doi: 10.1007/s00402-003-0537-5. [DOI] [PubMed] [Google Scholar]
- 160.Keller JC, Trancik TM, Young FA, St Mary E. Effects of indomethacin on bone ingrowth. J Orthop Res. 1989;7:28–34. doi: 10.1002/jor.1100070105. [DOI] [PubMed] [Google Scholar]
- 161.Trancik T, Mills W, Vinson N. The effect of indomethacin, aspirin, and ibuprofen on bone ingrowth into a porous-coated implant. Clin Orthop Relat Res. 1989;249:113–121. [PubMed] [Google Scholar]
- 162.Saudan M, Saudan P, Perneger T, Riand N, Keller A, Hoffmeyer P. Celecoxib versus ibuprofen in the prevention of heterotopic ossification following total hip replacement: a prospective randomised trial. J Bone Joint Surg Br. 2007;89:155–159. doi: 10.1302/0301-620X.89B2.17747. [DOI] [PubMed] [Google Scholar]
- 163.Bek D, Beksaç B, Della Valle AG, Sculco TP, Salvati EA. Aspirin decreases the prevalence and severity of heterotopic ossification after 1-stage bilateral total hip arthroplasty for osteoarthrosis. J Arthroplasty. 2009;24:226–232. doi: 10.1016/j.arth.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 164.Stoltny T, Koczy B, Wawrzynek W, Miszczyk L. Heterotopic ossification in patients after total hip replacement. Ortop Traumatol Rehabil. 2007;9:264–272. [PubMed] [Google Scholar]
- 165.Reddy MS, Jeffcoat MK, Richardson RC. Assessment of adjunctive flurbiprofen therapy in root-form implant healing with digital subtraction radiography. J Oral Implantol. 1990;16:272–276. [PubMed] [Google Scholar]
- 166.Alissa R, Sakka S, Oliver R, et al. Influence of ibuprofen on bone healing around dental implants: a randomised double-blind placebo-controlled clinical study. Eur J Oral Implantol. 2009;2:185–199. [PubMed] [Google Scholar]
- 167.Rainsford KD. Anti-inflammatory drugs in the 21st century. Sub-cell Biochem. 2007;42:3–27. doi: 10.1007/1-4020-5688-5_1. [DOI] [PubMed] [Google Scholar]
- 168.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18:790–804. doi: 10.1096/fj.03-0645rev. [DOI] [PubMed] [Google Scholar]
- 169.Kim DM, Koszeghy KL, Badovinac RL, Kawai T, Hosokawa I, Howell TH, et al. The effect of aspirin on gingival crevicular fluid levels of inflammatory and anti-inflammatory mediators in patients with gingivitis. J Periodontol. 2007;78:1620–1626. doi: 10.1902/jop.2007.070011. [DOI] [PubMed] [Google Scholar]
- 170.Offenbacher S, Odle BM, Van Dyke TE. The use of crevicular fluid prostaglandin E2 levels as a predictor of periodontal attachment loss. J Periodontal Res. 1986;21:101–112. doi: 10.1111/j.1600-0765.1986.tb01443.x. [DOI] [PubMed] [Google Scholar]
- 171.Inada M, Matsumoto C, Uematsu S, Akira S, Miyaura C. Membrane-bound prostaglandin E synthase-1-mediated prostaglandin E2 production by osteoblast plays a critical role in lipopolysaccharide-induced bone loss associated with inflammation. J Immunol. 2006;177:1879–1885. doi: 10.4049/jimmunol.177.3.1879. [DOI] [PubMed] [Google Scholar]
- 172.Pilbeam CC, Harrison JR, Raisz LG. Prostaglandins and bone metabolism. In: Bilezikian JP, Roasz LG, Rodan DA, editors. Principles of Bone Biology. San Diego, CA: Academic Press; 1996. pp. 715–728. [Google Scholar]
- 173.Ramirez-Yanez GO, Seymour GJ, Walsh LJ, Forwood MR, Symons AL. Prostaglandin E2 enhances alveolar bone formation in the rat mandible. Bone. 2004;35:1361–1368. doi: 10.1016/j.bone.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 174.Stichtenoth DO, Tsikas D, Gutzki FM, Frolich JC. Effects of ketoprofen and ibuprofen on platelet aggregation and prostanoid formation in man. Eur J Clin Pharmacol. 1996;51:231–234. doi: 10.1007/s002280050189. [DOI] [PubMed] [Google Scholar]
- 175.Kehoe MJ, Cohen SM, Zarrinnia K, Cowan A. The effect of acetaminophen, ibuprofen, and misoprostol on prostaglandin E2 synthesis and the degree and rate of orthodontic tooth movement. Angle Orthod. 1996;66:339–349. doi: 10.1043/0003-3219(1996)066<0339:TEOAIA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 176.Xiaoting L, Yin T, Yangxi C. Interventions for pain during fixed orthodontic appliance therapy. A systematic review. Angle Orthod. 2010;80:925–932. doi: 10.2319/010410-10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Oliver RG, Knapman YM. Attitudes to orthodontic treatment. Br J Orthod. 1985;12:179–188. doi: 10.1179/bjo.12.4.179. [DOI] [PubMed] [Google Scholar]
- 178.Scheurer PA, Firestone AR, Burgin WB. Perception of pain as a result of orthodontic treatment with fixed appliances. Eur J Orthod. 1996;18:349–357. doi: 10.1093/ejo/18.4.349. [DOI] [PubMed] [Google Scholar]
- 179.Soltis JE, Nakfoor PR, Bowman DC. Changes in ability of patients to differentiate intensity of forces applied to maxillary central incisors during orthodontic treatment. J Dent Res. 1971;50:590–596. doi: 10.1177/00220345710500031101. [DOI] [PubMed] [Google Scholar]
- 180.Jones ML. An investigation into the initial discomfort caused by placement of an archwire. Eur J Orthod. 1984;6:48–54. doi: 10.1093/ejo/6.1.48. [DOI] [PubMed] [Google Scholar]
- 181.Ngan P, Kess B, Wilson S. Perception of discomfort by patients undergoing orthodontic treatment. Am J Orthod Dentofacial Orthop. 1989;96:47–53. doi: 10.1016/0889-5406(89)90228-x. [DOI] [PubMed] [Google Scholar]
- 182.Krishnan V. Orthodontic pain: from causes to management – a review. Eur J Orthod. 2007;29:170–179. doi: 10.1093/ejo/cjl081. [DOI] [PubMed] [Google Scholar]
- 183.Profit WR. Contemporary Orthodontics. 3rd ed. St. Louis: Mosby Year Book; 2000. [Google Scholar]
- 184.Gurton AU, Akin E, Sagdic D, Olmez H. Effects of PGI2 and TxA2 analogs and inhibitors in orthodontic tooth movement. Angle Orthod. 2004;74:526–532. doi: 10.1043/0003-3219(2004)074<0526:EOPTAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 185.Sekhavat AR, Mousavizadeh K, Pakshir HR, Aslani FS. Effect of misoprostol, a prostaglandin E1 analog, on orthodontic tooth movement in rats. Am J Orthod Dentofacial Orthop. 2002;122:542–547. doi: 10.1067/mod.2002.126153. [DOI] [PubMed] [Google Scholar]
- 186.Yamasaki K, Shibata Y, Fukuhara T. The effect of prostaglandins on experimental tooth movement in monkeys (Macaca fuscata) J Dent Res. 1982;61:1444–1446. doi: 10.1177/00220345820610121401. [DOI] [PubMed] [Google Scholar]
- 187.Kale S, Kocadereli I, Atilla P, Assan E. Comparison of the effects of I,25 dihydroxycholecalciferol and prostaglandin E2 on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2004;125:607–614. doi: 10.1016/j.ajodo.2003.06.002. [DOI] [PubMed] [Google Scholar]
- 188.Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971;231:232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- 189.Sandy JR, Harris M. Prostaglandins and tooth movement. Eur J Orthod. 1984;6:175–182. doi: 10.1093/ejo/6.3.175. [DOI] [PubMed] [Google Scholar]
- 190.Kyrkanides S, O'Banion MK, Subtelny JD. Nonsteroidal anti-inflammatory drugs in orthodontic tooth movement: metalloproteinase activity and collagen synthesis by endothelial cells. Am J Orthod Dentofacial Orthop. 2000;118:203–209. doi: 10.1067/mod.2000.105872. [DOI] [PubMed] [Google Scholar]
- 191.Kehoe MJ, Cohen SM, Zarrinnia K, Cowan A. The effect of acetaminophen, ibuprofen, and misoprostol on prostaglandin E2 synthesis and the degree and rate of orthodontic tooth movement. Angle Orthod. 1996;66:339–349. doi: 10.1043/0003-3219(1996)066<0339:TEOAIA>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 192.Roche JJ, Cisneros GJ, Acs G. The effect of acetaminophen on tooth movement in rabbits. Angle Orthod. 1997;67:231–236. doi: 10.1043/0003-3219(1997)067<0231:TEOAOT>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 193.Arias OR, Marquez-Orozco MC. Aspirin, acetaminophen, and ibuprofen: their effects on orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 2006;130:364–370. doi: 10.1016/j.ajodo.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 194.Olson NZ, Otero AM, Marrero I, et al. Onset of analgesia for liquigel ibuprofen 400 mg, acetaminophen 1000 mg, ketoprofen 25 mg, and placebo in the treatment of postoperative dental pain. J Clin Pharmacol. 2001;41:1238–1247. doi: 10.1177/00912700122012797. [DOI] [PubMed] [Google Scholar]
- 195.Forbes JA, Kehm CJ, Grodin CD, Beaver WT. Evaluation of ketorolac, ibuprofen, acetaminophen, and an acetaminophen-codeine combination in postoperative oral surgery pain. Pharmacotherapy. 1990;10:94S–105S. [PubMed] [Google Scholar]
- 196.Cooper SA. Five studies on ibuprofen for postsurgical dental pain. Am J Med. 1984;77:70–77. doi: 10.1016/s0002-9343(84)80022-4. [DOI] [PubMed] [Google Scholar]
- 197.Minor V, Marris CK, McGorray SP, et al. Effects of preoperative ibuprofen on pain after separator placement. Am J Orthod Dentofacial Orthop. 2009;136:510–517. doi: 10.1016/j.ajodo.2007.09.018. [DOI] [PubMed] [Google Scholar]
- 198.Parameter on chronic periodontitis with slight to moderate loss of periodontal support. American Academy of Periodontology. J Periodontol. 2000;71(5 Suppl):853–855. doi: 10.1902/jop.2000.71.5-S.853. [DOI] [PubMed] [Google Scholar]
- 199.Parameter on periodontal maintenance. American Academy of Periodontology. J Periodontol. 2000;71(5 Suppl):849–850. doi: 10.1902/jop.2000.71.5-S.849. [DOI] [PubMed] [Google Scholar]
- 200.Needleman I, Suvan J, Moles DR, Pimlott J. A systematic review of professional mechanical plaque removal for prevention of periodontal diseases. J Clin Periodontol. 2005;32(Suppl 6):229–282. doi: 10.1111/j.1600-051X.2005.00804.x. [DOI] [PubMed] [Google Scholar]
- 201.Pihlstrom BL, Hargreaves KM, Bouwsma OJ, Myers WR, Goodale MB, Doyle MJ. Pain after periodontal scaling and root planing. J Am Dent Assoc. 1999;130:801–807. doi: 10.14219/jada.archive.1999.0303. [DOI] [PubMed] [Google Scholar]
- 202.van Steenberghe D, Garmyn P, Geers L, et al. Patients’ experience of pain and discomfort during instrumentation in the diagnosis and non-surgical treatment of periodontitis. J Periodontol. 2004;75:1465–1470. doi: 10.1902/jop.2004.75.11.1465. [DOI] [PubMed] [Google Scholar]
- 203.Hoffman A, Marshall RI, Bartold PM. Use of the vector scaling unit in supportive periodontal therapy: a subjective patient evaluation. J Clin Periodontol. 2005;32:1089–1093. doi: 10.1111/j.1600-051X.2005.00794.x. [DOI] [PubMed] [Google Scholar]
- 204.Kocher T, Rodemerk B, Fanghanel J, Meissner G. Pain during prophylaxis treatment elicited by two power-driven instruments. J Clin Periodontol. 2005;32:535–538. doi: 10.1111/j.1600-051X.2005.00707.x. [DOI] [PubMed] [Google Scholar]
- 205.Sachs CJ. Oral analgesics for acute nonspecific pain. Am Fam Physician. 2005;71:913–918. [PubMed] [Google Scholar]
- 206.Fornasini G, Monti N, Brogin G, et al. Preliminary pharmacokinetic study of ibuprofen enantiomers after administration of a new oral formulation (ibuprofen arginine) to healthy male volunteers. Chirality. 1997;9:297–302. [Google Scholar]
- 207.Black P, Max MB, Desjardins P, Norwood T, Ardia A, Pallotta T. A randomized, double-blind, placebo-controlled comparison of the analgesic efficacy, onset of action, and tolerability of ibuprofen arginate and ibuprofen in postoperative dental pain. Clin Ther. 2002;24:1072–1089. doi: 10.1016/s0149-2918(02)80020-0. [DOI] [PubMed] [Google Scholar]
- 208.Ettlin DA, Ettlin A, Bless K, et al. Ibuprofen arginine for pain control during scaling and root planing: a randomized, triple-blind trial. J Clin Periodontol. 2006;33:345–350. doi: 10.1111/j.1600-051X.2006.00918.x. [DOI] [PubMed] [Google Scholar]
- 209.Tam L. The safety of home bleaching techniques. J Can Dent Assoc. 1999;65:453–455. [PubMed] [Google Scholar]
- 210.Leonard RH, Jr, Haywood VB, Phillips C. Risk factors for developing tooth sensitivity and gingival irritation associated with night-guard vital bleaching. Quintessance Int. 1997;28:527–534. [PubMed] [Google Scholar]
- 211.Charakorn P, Cabanilla LL, Wagner WC, et al. The effect of preoperative ibuprofen on tooth sensitivity caused by in-office bleaching. OperDent. 2009;34:131–135. doi: 10.2341/08-33. [DOI] [PubMed] [Google Scholar]
- 212.Warner TD, Giuliano F, Vojnovic I, Bukasa A, Mitchell JA, Vane JR. Nonsteroid drug selectivities for cyclooxygenase-1 rather than cyclooxygenase-2 are associated with human gastrointestinal toxicity: a full in vitro analysis. Proc Natl Acad Sci U S A. 1999;96:7563–7568. doi: 10.1073/pnas.96.13.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Gallelli L, Colosimo M, Pirritano D, et al. Retrospective evaluation of adverse drug reaction induced by nonsteroidal anti-inflammatory drugs. Clin Drug Investig. 2007;27:115–122. doi: 10.2165/00044011-200727020-00004. [DOI] [PubMed] [Google Scholar]
- 214.Henry D, Lim LL, Garcia Rodriguez LA, et al. Variability in risk of gastrointestinal complications with individual non-steroidal anti-inflammatory drugs: results of a collaborative meta-analysis. BMJ. 1996;312:1563–1566. doi: 10.1136/bmj.312.7046.1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lugardon S, Lapeyre-Mestre M, Montastruc JL. Upper gastrointestinal adverse drug reactions and cyclooxygenase-2 inhibitors (celecoxib and rofecoxib): a case/non-case study from the French Pharmacovigilance Database. Eur J Clin Pharmacol. 2004;60:673–677. doi: 10.1007/s00228-004-0813-5. [DOI] [PubMed] [Google Scholar]
- 216.Moore N. Forty years of ibuprofen use. Int J Clin Pract Suppl. 2003:28–31. [PubMed] [Google Scholar]
- 217.Merlani G, Fox M, Oehen HP, et al. Fatal hepatoxicity secondary to nimesulide. Eur J Clin Pharmacol. 2001;57:321–326. doi: 10.1007/s002280100312. [DOI] [PubMed] [Google Scholar]
- 218.Rabkin JM, Smith MJ, Orloff SL, Corless CL, Stenzel P, Olyaei AJ. Fatal fulminant hepatitis associated with bromfenac use. Ann Pharmacother. 1999;33:945–947. doi: 10.1345/aph.18364. [DOI] [PubMed] [Google Scholar]
- 219.McCormick PA, Kennedy F, Curry M, Traynor O. COX-2 inhibitor and fulminant hepatic failure. Lancet. 1999;353:40–41. doi: 10.1016/s0140-6736(05)74867-4. [DOI] [PubMed] [Google Scholar]
- 220.Heard K, Green JL, Bailey JE, Bogdan GM, Dart RC. A randomized trial to determine the change in alanine aminotransferase during 10 days of paracetamol (acetaminophen) in subjects who consume moderate amounts of alcohol. Aliment Pharmacol Ther. 2007;26:283–290. doi: 10.1111/j.1365-2036.2007.03368.x. [DOI] [PubMed] [Google Scholar]
- 221.Gallelli L, Ferraro M, Mauro GF, De Fazio S, De Sarro G. Nimesulide-induced hepatotoxicity in a previously healthy woman. Clin Drug Investig. 2005;25:421–424. doi: 10.2165/00044011-200525060-00008. [DOI] [PubMed] [Google Scholar]
- 222.Heard K. Nonprescription analgesics: misunderstood and abused. Emergency Medicine. 2009;41:25–29. [Google Scholar]
- 223.FDA TAHWG. 2009 Joint Meeting of the Drug Safety and Risk Management Advisory Committee with the Anesthetic and Life Support Drugs Advisory Committee and the Nonprescription Drugs Advisory Committee. [accessed Aug 122009]. Http://www.fda.gov/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/DrugSafetyandRiskManagementAdvisoryCommittee/ucm161515.htm.
- 224.Graham GG, Hicks M. Pharmacokinetics and metabolism of paracetamol (acetaminophen) In: Rainsford KD, editor. Aspirin and Related Drugs. London: Taylor & Francis; 2004. pp. 182–213. [Google Scholar]
- 225.Doyle G, Furey S, Berlin R, et al. Gastrointestinal safety and tolerance of ibuprofen at maximum over-the-counter dose. Aliment Pharmacol Ther. 1999;13:897–906. doi: 10.1046/j.1365-2036.1999.00539.x. [DOI] [PubMed] [Google Scholar]
- 226.Rainsford KD, Roberts SC, Brown S. Ibuprofen and paracetamol: relative safety in non-prescription dosages. J Pharm Pharmacol. 1997;49:345–376. doi: 10.1111/j.2042-7158.1997.tb06809.x. [DOI] [PubMed] [Google Scholar]
- 227.Nimesulide ed epatotosicità. BIF. 2007;XIV:112–116. [Google Scholar]
- 228.Bunting S, Gryglewski R, Moncada S, Vane JR. Arterial walls generate from prostaglandin endoperoxides a substance (prostaglandin X) which relaxes strips of mesenteric and coeliac arteries and inhibits platelet aggregation. Prostaglandins. 1976;12:897–913. doi: 10.1016/0090-6980(76)90125-8. [DOI] [PubMed] [Google Scholar]
- 229.Fitzgerald GA, Smith B, Pedersen AK, Brash AR. Increased prostacyclin biosynthesis in patients with severe atherosclerosis and platelet activation. N Engl J Med. 1984;310:1065–1068. doi: 10.1056/NEJM198404263101701. [DOI] [PubMed] [Google Scholar]
- 230.Fitzgerald GA. Coxibs and cardiovascular disease. N Engl J Med. 2004;351:1709–1711. doi: 10.1056/NEJMp048288. [DOI] [PubMed] [Google Scholar]
- 231.Juni P, Nartey L, Reichenbach S, Sterchi R, Dieppe PA, Egger M. Risk of cardiovascular events and rofecoxib: cumulative meta-analysis. Lancet. 2004;364:2021–2029. doi: 10.1016/S0140-6736(04)17514-4. [DOI] [PubMed] [Google Scholar]
- 232.Chou R, Helfand M, Peterson K, Dana T, Roberts C. Comparative Effectiveness and Safety of Analgesics for Osteoarthritis (Internet) Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. Sep, [PubMed] [Google Scholar]
- 233.Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA American Heart Association. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007 Mar 27;115(12):1634–42. doi: 10.1161/CIRCULATIONAHA.106.181424. [DOI] [PubMed] [Google Scholar]
- 234.García Rodríguez LA, Hernández-Díaz S. Nonsteroidal antiinflammatory drugs as a trigger of clinical heart failure. Epidemiology. 2003;14:240–246. doi: 10.1097/01.EDE.0000034633.74133.C3. [DOI] [PubMed] [Google Scholar]
- 235.Curhan GC, Willett WC, Rosner B, Stampfer MJ. Frequency of analgesic use and risk of hypertension in younger women. Arch Intern Med. 2002;162:2204–2208. doi: 10.1001/archinte.162.19.2204. [DOI] [PubMed] [Google Scholar]
- 236.Chan AT, Manson JE, Albert CM, et al. Nonsteroidal antiinflammatory drugs, acetaminophen, and the risk of cardiovascular events. Circulation. 2006;113:1578–1587. doi: 10.1161/CIRCULATIONAHA.105.595793. [DOI] [PubMed] [Google Scholar]
- 237.Rahme E, Nedjar H. Risks and benefits of COX-2 inhibitors vs non-selective NSAIDs: does their cardiovascular risk exceed their gastrointestinal benefit? A retrospective cohort study. Rheumatology (Oxford) 2007;46:435–438. doi: 10.1093/rheumatology/kel428. [DOI] [PubMed] [Google Scholar]
- 238.Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004 Feb 28;363(9410):717–27. doi: 10.1016/S0140-6736(04)15648-1. [DOI] [PubMed] [Google Scholar]
- 239.Raisz LG. Potential impact of selective cyclooxygenase-2 inhibitors on bone metabolism in health and disease. Am J Med. 2001;110(Suppl 3A):43S–45S. doi: 10.1016/s0002-9343(00)00684-7. [DOI] [PubMed] [Google Scholar]
- 240.Radi ZA, Khan NK. Effects of cyclooxygenase inhibition on bone, tendon and ligament healing. Inflamm Res. 2005;54:358–366. doi: 10.1007/s00011-005-1367-4. [DOI] [PubMed] [Google Scholar]
- 241.Huo MH, Troiano NW, Pelker RR, Gundberg CM, Friedlaender GE. The influence of ibuprofen on fracture repair: biomechanical, biochemical, histological and histomorphometric parameters in rats. J Orthop Res. 1991;9:383–390. doi: 10.1002/jor.1100090310. [DOI] [PubMed] [Google Scholar]
- 242.Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am. 2004;86-A:116–123. doi: 10.2106/00004623-200401000-00017. [DOI] [PubMed] [Google Scholar]
- 243.Seidenberg AB, An YH. Is there an inhibitory effect of COX-2 inhibitors on bone healing? Pharmacol Res. 2004;50:151–156. doi: 10.1016/j.phrs.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 244.Brocks D, Jamali F. The pharmacokinetics of ibuprofen in humans and animals. In: Rainsford KD, editor. Ibuprofen. A critical Bibliographic Review. London: Taylor & Francis; 1999. pp. 89–142. [Google Scholar]
- 245.Tornio A, Niemi M, Neuvonen PJ, Backman JT. Stereoselective interaction between the CYP2C8 inhibitor gemfibrozil and racemic ibuprofen. Eur J Clin Pharmacol. 2007;63:463–469. doi: 10.1007/s00228-007-0273-9. [DOI] [PubMed] [Google Scholar]
- 246.Werner U, Werner D, Rau T, Fromm MF, Hinz B, Brune K. Celecoxib inhibits metabolism of cytochrome P450 2D6 substrate metoprolol in humans. Clin Pharmacol Ther. 2003;74:130–137. doi: 10.1016/S0009-9236(03)00120-6. [DOI] [PubMed] [Google Scholar]
- 247.Davies NM, Anderson KE. Clinical pharmacokinetics of diclofenac. Therapeutic insights and pitfalls. Clin Pharmacokinet. 1997;33:184–213. doi: 10.2165/00003088-199733030-00003. [DOI] [PubMed] [Google Scholar]
- 248.Jamali F, Brocks DR. Clinical pharmacokinetics of ketoprofen and its enantiomers. Clin Pharmacokinet. 1990;19:197–217. doi: 10.2165/00003088-199019030-00004. [DOI] [PubMed] [Google Scholar]
- 249.Igbal Z, Khan A, Naz A, Khan JA, Khan GS. Pharmacokinetic interaction of ciprofloxacin with diclofenac: a single-dose, two-period crossover study in healthy adult volunteers. Clin Drug Investig. 2009;29:275–281. doi: 10.2165/00044011-200929040-00006. [DOI] [PubMed] [Google Scholar]
- 250.Karjalainen MJ, Neuvonen PJ, Backman JT. In vitro inhibition of CYP1A2 by model inhibitors, anti-inflammatory analgesics and female sex steroids: predictability of in vivo interactions. Basic Clin Pharmacol Toxicol. 2008;103:157–165. doi: 10.1111/j.1742-7843.2008.00252.x. [DOI] [PubMed] [Google Scholar]
- 251.Khamdang S, Takeda M, Noshiro R, et al. Interactions of human organic anion transporters and human organic cation transporters with nonsteroidal anti-inflammatory drugs. J Pharmacol Exp Ther. 2002;303:534–539. doi: 10.1124/jpet.102.037580. [DOI] [PubMed] [Google Scholar]
- 252.Shitara Y, Horie T, Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur J Pharm Sci. 2006;27:425–446. doi: 10.1016/j.ejps.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 253.Catella-Lawson F, Reilly MP, Kapoor SC, et al. Cyclooxygenase inhibitors and the antiplatelet effects of aspirin. N Engl J Med. 2001;345:1809–1817. doi: 10.1056/NEJMoa003199. [DOI] [PubMed] [Google Scholar]
- 254.Kimmel SE, Berlin JA, Reilly M, et al. The effects of nonselective non-aspirin non-steroidal anti-inflammatory medications on the risk of nonfatal myocardial infarction and their interaction with aspirin. J Am Coll Cardiol. 2004;43:985–990. doi: 10.1016/j.jacc.2003.08.064. [DOI] [PubMed] [Google Scholar]
- 255.Curtis JP, Wang Y, Portnay EL, Masoudi FA, Havranek EP, Krumholz HM. Aspirin, ibuprofen, and mortality after myocardial infarction: retrospective cohort study. BMJ. 2003;327:1322–1323. doi: 10.1136/bmj.327.7427.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Kean WF, Rainsford KD, Kean IR. Management of chronic musculoskeletal pain in the elderly: opinions on oral medication use. Inflammopharmacology. 2008;16:53–75. doi: 10.1007/s10787-008-1623-7. [DOI] [PubMed] [Google Scholar]
- 257.Cryer B, Berlin RG, Cooper SA, Hsu C, Wason S. Double-blind, randomized, parallel, placebo-controlled study of ibuprofen effects on thromboxane B2 concentrations in aspirin-treated healthy adult volunteers. Clin Ther. 2005;27:185–191. doi: 10.1016/j.clinthera.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 258.Schuijt MP, Huntjens-Fleuren HW, de Metz M, Vollaard EJ. The interaction of ibuprofen and diclofenac with aspirin in healthy volunteers. Br J Pharmacol. 2009;157:931–934. doi: 10.1111/j.1476-5381.2009.00243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.http://www.fda.gov/medwatch/report.htm.
- 260.Grennan DM, Ferry DG, Ashworth ME, Kenny RE, Mackinnon M. The aspirin-ibuprofen interaction in rheumatoid arthritis. Br J Clin Pharmacol. 1979;8:497–503. doi: 10.1111/j.1365-2125.1979.tb01033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Velo GP, Minuz P, Arosio E, Capuzzo MG, Covi G, Lechi A. Interaction between non-steroidal anti-inflammatory drugs and angiotensin-converting enzyme inhibitors in man. In: Rainsford KD, Velo GP, editors. Side-Effects of Anti-inflammatory Drugs. Part 1. Clinical and Epidemiological Aspects. Lancaster (UK): MTP Press; 1987. pp. 195–201. [Google Scholar]
- 262.Badin C, Chambrier C, Anouifi A, Boucaud C, Bouletreau P. Non-steroidal anti-inflammatory agents and angiotensin converting enzyme inhibitor: a dangerous combination during postoperative period. Ann Fr Anesth Reanim. 1997;16:55–57. doi: 10.1016/s0750-7658(97)84279-7. [DOI] [PubMed] [Google Scholar]
- 263.Wolf K, Castrop H, Hartner A, Goppelt-Strübe M, Hilgers K, Kurtz A. Inhibition of the rennin-angiotensin system upregulates cyclooxygenase-2 expression in the macula densa. Hypertension. 1999;34:503–507. doi: 10.1161/01.hyp.34.3.503. [DOI] [PubMed] [Google Scholar]
- 264.Miwa LJ, Jones JK. Adverse drug reactions attributed to ibuprofen: effects other than gastrointestinal. Ibuprofen: A critical Bibliographic Review. In: Rainsford KD, editor. London: Taylor & Francis; 1999. pp. 499–538. [Google Scholar]