Fig. 2.
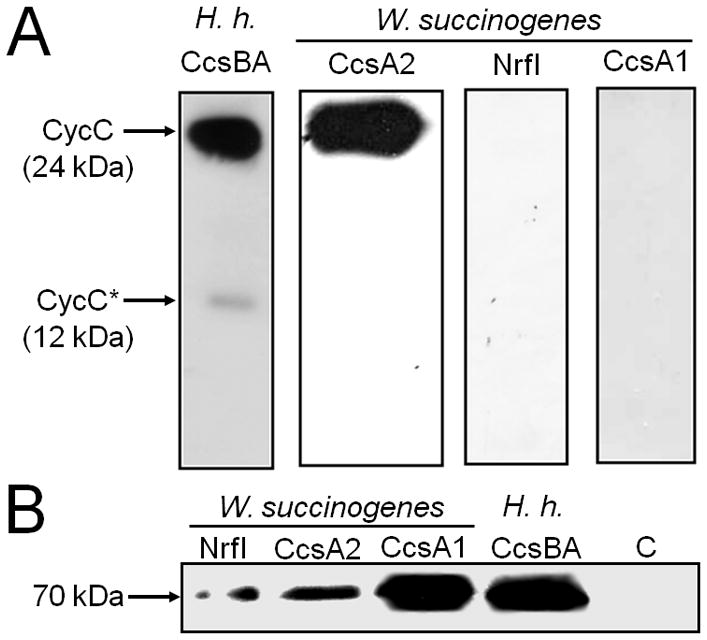
Production of CCHL isoenzymes from W. succinogenes in E. coli RK103 and their functionality in haem c attachment to a reporter cytochrome with two CX2CH haem c binding motifs. E. coli strains 21–24 (Table 4) were used.
A. Chemiluminescent haem stain detection of B. pertussis CycC in periplasmic extracts (35 μg per lane) of E. coli RK103 producing the indicated CCHL. The cells contained a plasmid that enabled production of the indicated CCHL. The previously observed proteolytic degradation product of CycC (denoted CycC*) was only observed in cells harbouring the H. hepaticus (H.h.) CCHL (Richard-Fogal et al., 2007).
B. Immunoblot detection of indicated CCHLs by Western blot analysis using an anti-GST serum. 50 μg of total cell protein were applied to each lane. The detected proteins of around 70 kDa most likely represent proteolytic CCHL fragments, each comprising the GST tag and the CcsB part of the CcsBA fusion protein. C refers to a control experiment using E. coli RK103 cells that contained the empty vectors pRGK330 and pGEX-4T-1 (Feissner et al., 2006). H.h., H. hepaticus.
