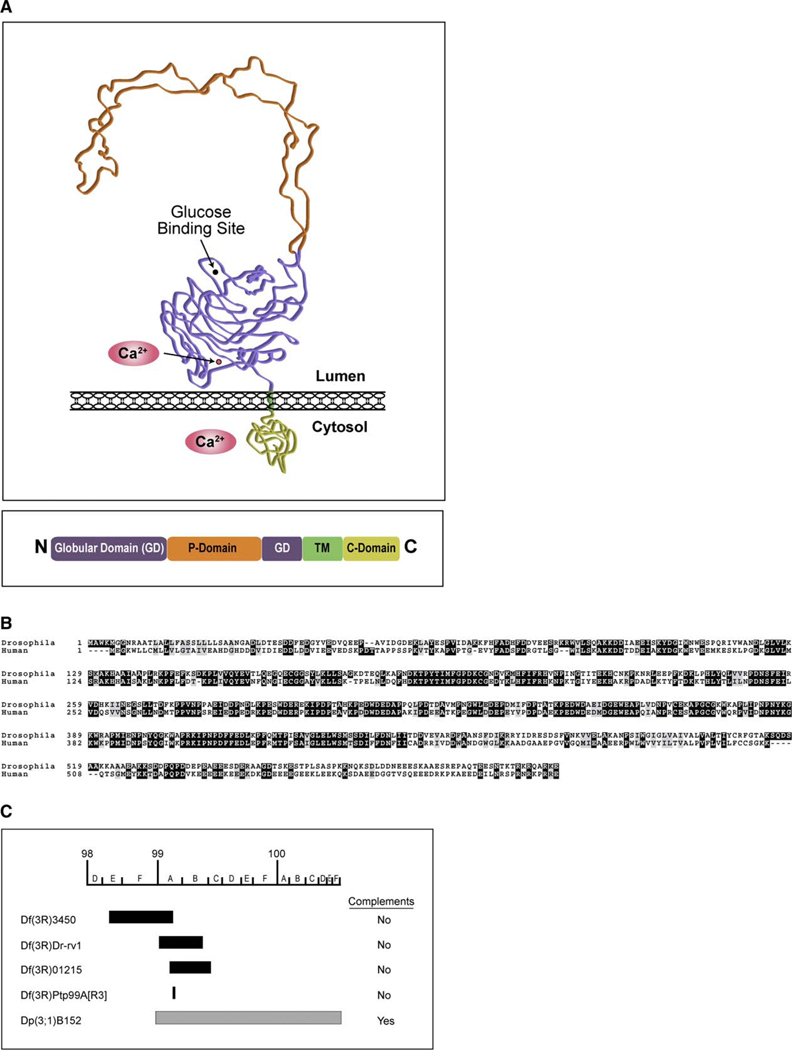
Figure 1. Drosophila Calnexin.
(A) Proposed structure of Cnx. (Top box) A schematic of the proposed three-dimensional structure for Cnx based on crystallization of the luminal domain of canine calnexin (Schrag et al., 2001). (Bottom box) The proposed domain structure of the Drosophila Cnx protein, showing the N-terminal luminal domains (amino acid numbers 1–489), the transmembrane (TM) domain (amino acid numbers 490–508), and the cytosolic domain (amino acid numbers 509–605). It has been proposed that the luminal domain contains two distinct regions: a compact, globular domain (purple) and a proline-rich arm called the P domain (orange) (Schrag et al., 2001). Shown are the proposed Ca2+-binding (Ca2+) and glucose-binding sites within the globular domain. The TM segment (green) was identified based on hydropathy plot analysis of the conceptual Cnx protein using the Kyte-Doolittle algorithm (Kyte and Doolittle, 1982) (data not shown). The carboxyl-terminal cytosolic domain (yellow) is highly charged and effectively binds Ca2+ (Tjoelker et al., 1994).
(B) Drosophila cnx encodes a 605 amino acid protein that displays 49% identity with human calnexin (chromosome 5q). Clustal/W amino acid alignment between Drosophila Cnx and human calnexin (CNX). Dark shading indicates identity, whereas light shading indicates similarity. Numbers refer to amino acids.
(C) Drosophila deficiencies and duplications corresponding to cnx are shown. The following deficiencies failed to complement the cnx alleles: Df(3R)3450 uncovers 98E3 to 99A6-8, Df(3R)Dr-rv1 uncovers 99A1-2 to 99B6-11, Df(3R)01215 uncovers 99A6 to 99C1, Df(3R)Ptp99A[R3] uncovers 99A7. A Drosophila stock with a duplication for 98F14 to 100F and translocation to the X chromosome [Dp(3;1)B152] positively complemented the cnx alleles.