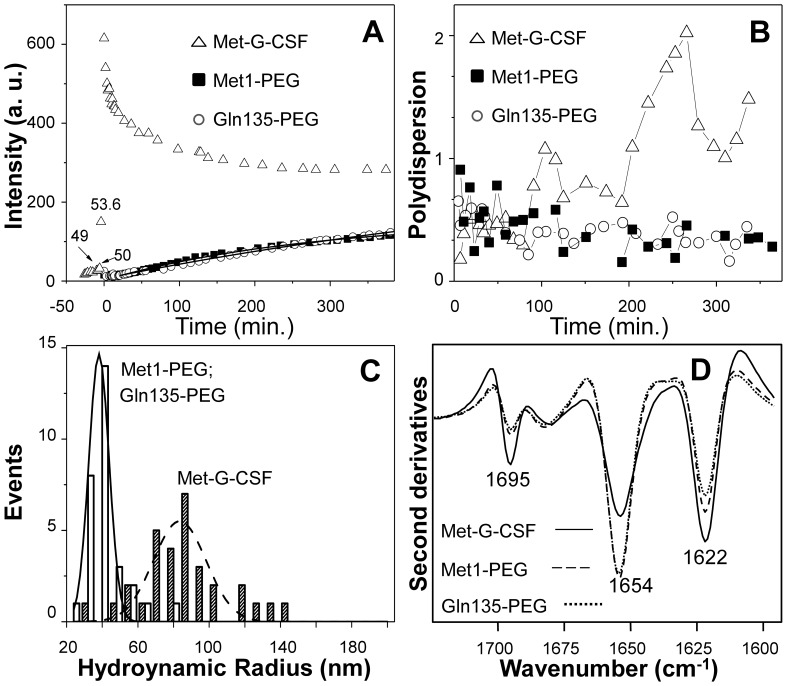
Figure 5. Dynamic light scattering and second derivative FTIR spectra of non-pegylated Met-G-CSF and of the two isomeric Met-G-CSF-Met1-PEG and Met-G-CSF-Gln135-PEG.
A) Light scattering intensity, in arbitrary units, as a function of the incubation time at 55°C. At the zero time the bath temperature was changed from 37°C to 55°C. B) Polydispersity index (Eq.3) of the non-pegylated Met-G-CSF and of the two isomeric pegylated proteins as a function of the incubation time. C) Distribution of the hydrodynamic radii corresponding to the most abundant component in the DLS decay. The solid and dashed lines are best fit Gaussian function to the data that corresponds to Rh = 39.4±4 nm and Rh = 70±20 nm for the pegylated and non-pegylated samples respectively. D) Met-G-CSF, Met-G-CSF-Met1-PEG and Met-G-CSF-Gln135-PEG second derivative FTIR spectra measured after 7 hours of incubation at 55°C.Concerning the size of the protein aggregates, the diffusion coefficients (Eq.1) were used to evaluate the protein aggregate hydrodynamic radii through Eq.2. Non-pegylated Met-G-CSF displayed an average hydrodynamic radius of 1.4±0.4 nm at 37°C with negligible presence of protein aggregates. At 55°C, after thermal equilibrium was reached, we found instead a prevalent component with Rh = 70±20 nm. It should be noted that the value of 70 nm represents the average size of protein aggregates still in solution after 7 hours of incubation at 55oC.