Abstract
The Mobile River Basin is a hotspot of molluscan endemism, but anthropogenic activities have caused at least 47 molluscan extinctions, 37 of which were gastropods, in the last century. Nine of these suspected extinctions were in the freshwater gastropod genus Leptoxis (Cerithioidea: Pleuroceridae). Leptoxis compacta, a Cahaba River endemic, has not been collected for >70 years and was formally declared extinct in 2000. Such gastropod extinctions underscore the imperilment of freshwater resources and the current biodiversity crisis in the Mobile River Basin. During a May 2011 gastropod survey of the Cahaba River in central Alabama, USA, L. compacta was rediscovered. The identification of snails collected was confirmed through conchological comparisons to the L. compacta lectotype, museum records, and radulae morphology of historically collected L. compacta. Through observations of L. compacta in captivity, we document for the first time that the species lays eggs in short, single lines. Leptoxis compacta is restricted to a single location in the Cahaba River, and is highly susceptible to a single catastrophic extinction event. As such, the species deserves immediate conservation attention. Artificial propagation and reintroduction of L. compacta into its native range may be a viable recovery strategy to prevent extinction from a single perturbation event.
Introduction
The Mobile River Basin (MRB) in Alabama and Georgia contains the highest levels of freshwater molluscan biodiversity in North America [1], [2], [3], [4]. Anthropogenic activities, however, have caused massive declines in gastropod biodiversity throughout the basin. At least 47 molluscan extinctions (37 gastropods) and many other local extirpations were the immediate result of inundation for hydropower, channelization for navigation and pollution from mine and urban centers throughout the mid 20th century [3], [4], [5]. These extinctions comprise a third of known molluscan extinctions globally [6], making the MRB a major component of the global biodiversity crisis.
Freshwater gastropods in the family Pleuroceridae (Gastropoda: Cerithioidea), suffered the largest number of the aforementioned MRB extinctions (∼29) [4]. Of the 14 native MRB Leptoxis species, nine are considered extinct, including L. compacta [7]. Four of the remaining five Leptoxis are classified under the U.S. Endangered Species Act as either threatened or endangered [8], [9]. Remaining Leptoxis species in the MRB are of high conservation concern, and they are the focus of active propagation and reintroduction efforts [10], [11], [12].
Leptoxis compacta was formally declared extinct in 2000 [13], and was not collected in a 1992 survey for the US Fish and Wildlife Service (USFWS) [14], a 2005 ADCNR survey of the Cahaba River [15]or in a more recent survey by Tolley-Jordan [16]. It is the only pleurocerid endemic to the Cahaba River considered extinct [7], [13], and has not been collected in at least 70 years. Historically, L. compacta was most abundant in the central section of the Cahaba River at Lily Shoals in Bibb County, Alabama [17], [18]. Exact causes of L. compacta's decline are unknown, but the species was declining in abundance and range by 1935 [17]. The snail's decline was likely a result of its naturally small range, pollution from mines in the area, and effluent from the Birmingham, Alabama metropolitan area [19].
In this study, we describe the results of targeted surveys for L. compacta in the middle portion of the Cahaba River in Bibb and Shelby Counties, Alabama. Whenever an “extinct” species is putatively re-discovered, special care must be taken to confirm the identity of the species [20]. As such, the conchological and radular morphology of L. compacta individuals collected in this study are compared to historically collected material along with other sympatric pleurocerids to confirm the identity and distinctness of L. compacta. We also document for the first time, the egg-laying strategy, juvenile morphology, and soft tissue pigmentation of L. compacta. Potential threats to the long-term survival of L. compacta are also discussed.
Results
Survey
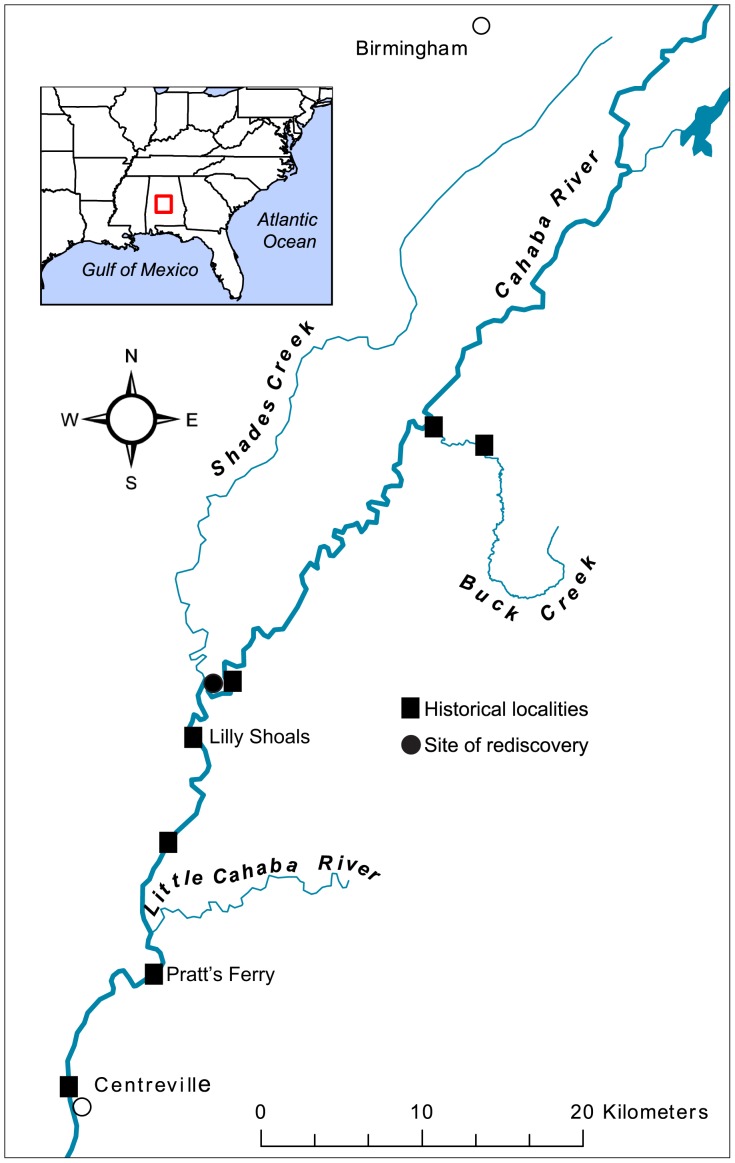
Museum lots of L. compacta reviewed are listed in Table 1 and historical localities are labeled on Figure 1. The historical range of L. compacta extended from Centerville, Bibb County, Alabama, USA upriver and into lower Buck Creek in the Valley and Ridge physiogeographic province of the southern Appalachian Mountains. The most recent lots we analyzed were from 1933 (UMMZ 57871, MCZ 98217), and as far as we are aware this was the last time the species was collected.
Table 1. Locality and museum catalogue numbers for L. compacta museum lots analyzed in this study.
| Locality | Catalogue # |
| Alabama (river unspecified), Lectotype | MCZ 072063 |
| Buck Creek | FLMNH 81147; USNM 158595 |
| Cahaba River (location unspecified) | USNM 15874, 218694, 158741, 509539*, 158743, 407631; UMMZ 55741 |
| Cahaba River at Abita | USNM 321957 |
| Cahaba River at Centerville | UMMZ 57871 |
| Cahaba River at Lily Shoals | FLMNH 81172, 81188, 81144, 7136; USNM 590380 |
| Cahaba River near Piper | FLMNH 81137, 81178 |
| Cahaba River at Pratt's Ferry | FLMNH 81165, MCZ 98217 |
Lot from which radulae were taken.
Figure 1. Map of the Cahaba River and select tributaries.
Historical collections sites were sampled in August 2011, but L. compacta was found only at the site of re-discovery.
Leptoxis compacta was found during the May 2011 survey on an unnamed shoal upstream of the Cahaba River and Shades Creek confluence in Shelby County Alabama (Fig. 1; 33.1786°N, 87.0174°W). At this site, we found every pleurocerid species known from the middle Cahaba River including the federally threatened Round Rocksnail, L. ampla (Anthony 1855) (Table 2) [17], [21]. We also found the endemic limpet Rhodacmea cahawbensis Walker, 1917 (Planorbidae) and Lepyrium showalteri (Lea, 1861) (Lithoglyphidae), which is federally endangered.
Table 2. Species collected in the May 2011 survey at the site of L. compacta re-discovery (33.178601°N, 87.017481°W).
| Species | Catalogue # |
| Elimia annettae | USNM 1186562 |
| Elimia ampla | USNM 1186561 |
| Elimia cahawbensis | USNM 1186563 |
| Elimia clara | USNM 1186564 |
| Elimia showalteri | USNM 1186566 |
| Elimia variata | USNM 1186567 |
| Leptoxis ampla | USNM 1186568 |
| Leptoxis compacta | USNM 1186565 FLMNH 449320 |
| Pleurocera prasinatum | USNM 1186569 |
| Rhodacmea cahabensis | USNM 1186570 |
Lepyrium showalteri was found, but not collected due its endangered status under the U.S. Endangered Species Act.
During the first survey we failed to locate L. compacta below the Shades Creek confluence. Furthermore, the second survey for L. compacta (Fig. 1) also failed to locate the species from other historical sites. At every site L. compacta was historically found that we sampled, other pleurocerid species were present.
Life history and morphology
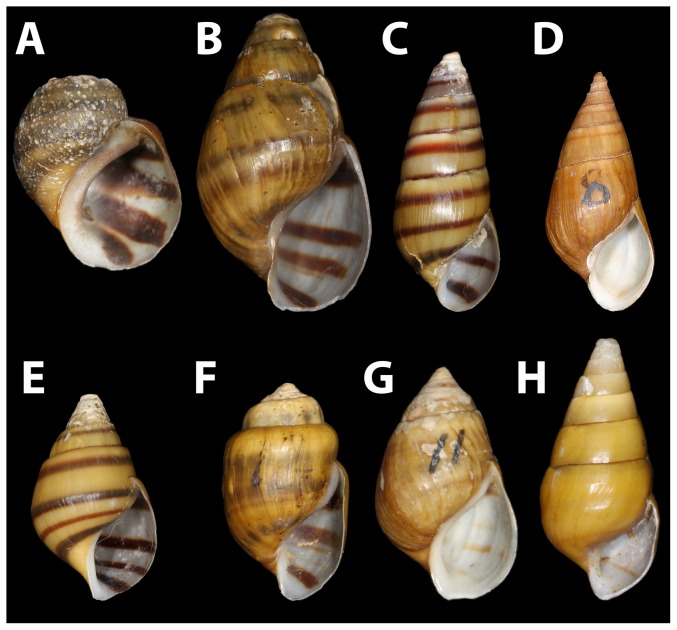
Snails we collected and putatively identified as L. compacta possess shells nearly identical to the lectotype (Fig. 2) and the original species description [22]. Leptoxis compacta shells do not closely resemble those of other sympatric species (Figs. 2, 3). Juvenile L. compacta shells possess one distinct carina on the main body whorl, which is eventually lost as adults (Fig. 2). Individuals with shell pigmentation lines are always present in three wide bands. Most wild-caught individuals had purple pigmentation on the columella indentation, but this trait was not observed in juveniles propagated in captivity. The external tissue pigmentation of L. compacta is yellow, mottled with black and includes prominent black bands in the middle of the proboscis and on both eyes (Fig. 4). This pigmentation banding pattern is identical to sympatric L. ampla (not figured). Pigmentation patterns and the presence of an ocular peduncle are features that distinguish L. compacta from sympatric Elimia spp. including E. clara (Anthony, 1854) (Fig. 5), which is conchologically most similar to L. compacta (Figs. 2, 3).
Figure 2. Growth series of L. compacta.
A) L. compacta lectotype (MCZ 072063). B–E) Wild caught individuals. F) Juvenile grown in culture, approximately 4 months old. Scale bar = 5 mm.
Figure 3. Lectotypes of pleurocerids sympatric with L. compacta.
A) L. ampla (MCZ 161803). B) E. ampla (MCZ 161735). C) E. annetae (UMMZ 128908). D) E. cahawbensis (USNM 119055). E) E. clara (MCZ 072329) F) E. showalteri (ANSP 26881) G) E. variata (USNM 118756). F) P. prasinatum (USNM 122206).
Figure 4. Photograph of live L. compacta from the Cahaba River, Shelby County, Alabama.
Figure 5. Photograph of live E. clara from the Cahaba River, Shelby County, Alabama.
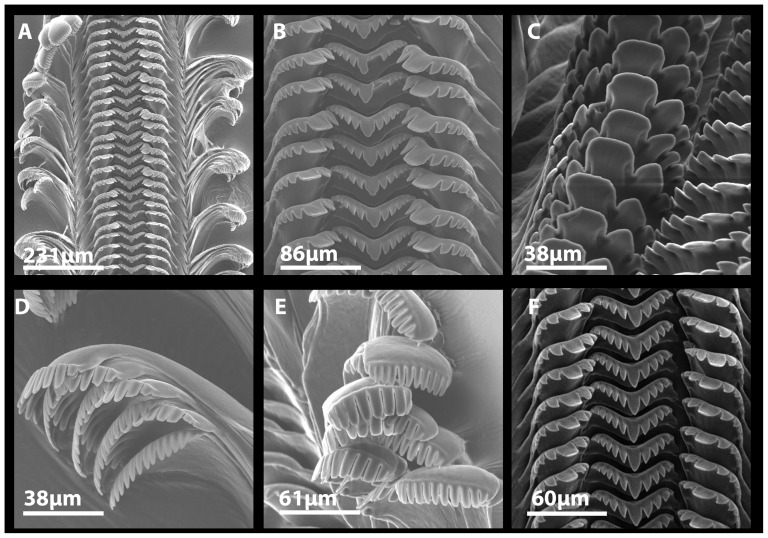
The radular structure of L. compacta specimens collected in May 2011 is identical to that of individuals collected in 1881 (Fig. 6). The basal margin of the rachidian tooth is widely convex. The central cusp is blunt and flanked by 4–5 denticles, with the outermost being weakly developed in most cases (Fig. 6 A, B). The lateral tooth contains one larger rectangular central cusp that is flanked by 4–5 outer denticles and 3–4 inner denticles (Fig. 6 B, C). The inner marginal teeth contain 10–12 denticles (Fig. 6 D, E). The number of denticles on the outer marginal teeth varies from 12–16, within and among individuals, and the outer denticles are often weakly developed (Fig. 6D, E).
Figure 6. Scanning electron micrographs of L. compacta radulae collected in May 2011 (A–E) and the radula of historically collected individual (F).
A) Section of anterior radular ribbon. B) Detailed view of rachidian and lateral teeth. C) View of lateral teeth at 45 degree angle, and slightly rotated counter-clockwise. D–E) Views of marginal teeth showing variation between individuals. F) Rachidian and lateral teeth removed from individual collected in 1881 (USNM 509539).
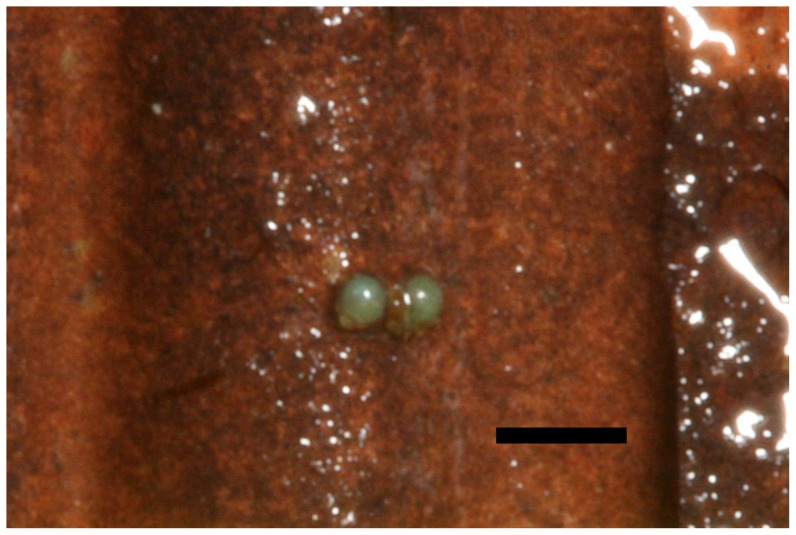
Eggs were laid by female L. compacta within three days of being transferred into captive culture. This suggests female snails were laying eggs in the wild when collected in May 2011. Oviposition ceased when the daily maximum water temperature reached 29°C. Eggs were laid either singly or in a linear sequence (Fig. 7). Each egg was approximately 0.3 mm in diameter. Average length of the line of eggs was 1.57 eggs (n = 51 egg lines) with a maximum observed length of 3 eggs.
Figure 7. Photograph of two eggs that were laid by captive L. compacta.
Scale bar = 1 mm.
Discussion
Only two types of morphological data are available to confirm the putative re-discovery of L. compacta: shell and radular. The primary type, shell descriptions in taxonomic works [18], [22] and museum records match the L. compacta we collected. Furthermore, radulae we extracted from L. compacta collected in 1881 are identical to those of live L. compacta collected in this study (Figure 6). Compared to sympatric pleurocerids, L. compacta shells most resemble those of Cahaba River Elimia spp. (Figs. 2, 3). Furthermore, the radular morphology is more similar to those of sympatric Elimia spp. than L. ampla [23], [24]. However, body pigmentation of L. compacta is most similar to that of L. ampla rather than sympatric Elimia (Figs. 4, 5). The aforementioned features of L. compacta are similar in some regards to both sympatric Elimia spp. and L. ampla, but taken in total distinguish L. compacta as unique. Molecular systematic analyses are underway to clarify the genetic position of L. compacta.
The reduced range of L. compacta qualifies the species as critically endangered under International Union for the Conservation of Nature criteria [25]. It is unclear why L. compacta suffered such a drastic range reduction while other sympatric pleurocerid species did not [15], [17]. However, point-source pollution and urban runoff from the Birmingham, Alabama metropolitan area threaten the long-term survival of L. compacta. Furthermore, the lone population of L. compacta is found adjacent to a large youth camp currently under construction. The U.S. Fish and Wildlife Service should consider L. compacta for protection under the U.S. Endangered Species Act because of its highly restricted range and susceptibility to a single pollution or siltation event. The ADCNR has already undertaken culturing projects for federally endangered L. plicata and L. foremani to expand their current range [10], [11], [12], and we argue that similar efforts should be pursued for L. compacta.
There is little literature on the reintroduction of freshwater gastropods [26], but general conservation rules [25], [27] and guidelines for reintroduction of fish are applicable for freshwater snails [28], [29], [30]. There are two sites within the historical range of L. compacta that we consider potentially viable for the reestablishment of a second population. Lily Shoals, which is isolated from development or bridge crossings, is 5.8KM downstream of the remaining L. compacta population and supports at least five other species of pleurocerids [17]. The second potential site for reintroduction is 25.8KM downstream of the site of rediscovery at Pratt's Ferry. Downstream sites are ideal for establishing a second L. compacta population because pleurocerid snails have a net upstream movement, thus have potential to naturally colonize upstream localities [31], [32]. These localities are also better suited than upstream sites because primary production is generally higher downstream [33]. Furthermore, the lone tributary that historically harbored L. compacta, Buck Creek, has three wastewater discharge points and suboptimal habitat [34], [35]. Extensive assessments should be performed to identify additional sites for reintroduction that will enhance the survival prospects of propagated L. compacta [11], [36].
Through comparisons to the L. compacta lectotype, and radulae from fresh and historic collections we present compelling evidence that L. compacta has been “re-discovered” in the Cahaba River in Shelby County, Alabama. Why three previous surveys of the Cahaba River—including the site of re-discovery—failed to locate L. compacta is unknown [14], [15], [16]. Because of its restricted range, L. compacta should be the focus of immediate conservation attention. Nevertheless, the rediscovery of L. compacta is an encouraging moment in the recent history of conservation and biodiversity studies of freshwater mollusks in the MRB.
Materials and Methods
Survey
Alabama Department of Conservation and Natural Resources scientific collecting permits and U.S. Fish and Wildlife Service permits for threatened species were obtained prior to sampling. Since L. compacta does not have a formal status under the U.S. Endangered Species Act, federal permit authorization does not apply.
To document the historical range of L. compacta and the approximate last collection of the species, museum specimens were analyzed at the National Museum of Natural History (NMNH), North Carolina Museum of Natural Sciences (NCMNS), and the Florida Museum of Natural History (FLMNH). Photographs of L. compacta lots housed at the Museum of Comparative Zoology at Harvard University (MCZ) and the University of Michigan Museum of Zoology (UMMZ) were also examined (Table 1). Spurious localities represented by only one lot and outside the otherwise documented range of L. compacta (e.g. “Alabama River at Selma,” USNM 178542) were excluded from consideration.
In May 2011 gastropods were qualitatively surveyed from a kayak in the Cahaba River (Fig. 1) from sites upstream of Shades Creek and Cahaba River confluence to below Piper Bridge. All other sites where L. compacta (Fig. 1) was historically found were surveyed in August 2011 to confirm range contraction of L. compacta. Live snails collected in May 2011 were transported to the Alabama Aquatic Biodiversity Center in Marion, Alabama for species identifications and preservation. Endangered species that we encountered were not collected. Identification of each species was based on comparison with primary types (Fig. 2), museum records, and descriptive literature [18], [21], [22], [37]. Snails collected in these surveys were preserved following Fukuda et al. [38] and deposited at NMNH and FLMNH (Table 2).
Morphological and life history analyses
A size range of L. compacta individuals was collected from the site of re-discovery, and live L. compacta were photographed in an aquarium and compared to other sympatric pleurocerids. We extracted radulae from two L. compacta specimens collected in the original May 2011 survey and from two samples of dried tissue left in shells from individuals collected in 1881 (USNM 509539). Radulae were extracted following the procedure of Holznagel [39]. Leptoxis compacta radulae were visualized on a Hitachi S-2599 Scanning Electron Microscope at the University of Alabama Optical Analysis Facility.
Approximately thirty L. compacta individuals were placed in captivity at the Alabama Aquatic Biodiversity Center to observe egg-laying behavior. Because of the difficulties of recording egg-laying strategies in the wild, a culturing environment is ideal for these observations [40]. Snails were kept in a 20 L acrylic tank with a 0.83 cm bulkhead fitting that allowed a constant exchange of thermally ambient well water. A 15 L/min powerhead was attached to the lid of the tank to create a constant flow regime. An airstone was placed in the tank to saturate the water with dissolved oxygen. Water temperature was measured hourly with a Hobo Temp Logger (Onset Computer Corporation).
At least every three days, tanks were checked for eggs and the eggs were counted and the adjacent area of the tank marked with a permanent marker to insure individual eggs were not counted twice. Juveniles were allowed to grow in the culturing environment for 5.5 months. Growth series of wild caught and cultured snails were compared to demonstrate conchological changes during ontogeny.
Acknowledgments
Thanks to E.E. Strong, A.E. Bogan, J. Slapcinsky, for access to collections. A. Baldinger and T. Lee provided photographs of lots at MCZ and UMMZ respectively. J. Pfeiffer, M.S. Piteo, and A.W. Whelan aided with field work. E.E. Strong and K. Lackey aided with SEM analysis. T. Tarpley took shell and live snail photographs. D.M Schneider and A. Wynn aided with GIS. D.L. Graf provided advice and use of lab space. Two anonymous reviewers and J.T. Garner helped to improve the manuscript from a previous version.
Funding Statement
Culturing of L. compacta was done under a State Wildlife Grant Agreement with the U.S. Fish and Wildlife Service and the Alabama Department of Conservation and Natural Resources. The University of Alabama Department of Biological Sciences and Conchologists of America provided grants to N.V. Whelan for fieldwork and SEM work. No additional external funding was received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Williams JD, Bogan AE, Garner JT (2008) Freshwater mussels of Alabama and the Mobile Basing in Georgia, Mississippi, and Tennessee. Tuscaloosa: University of Alabama Press. [Google Scholar]
- 2. Lydeard C, Mayden RL (1995) A Diverse and Endangered Aquatic Ecosystem of the Southeast United States. Conservation Biology 9: 800–805. [Google Scholar]
- 3.Neves RJ, Bogan AE, Williams JD, Ahlstedt SA, Hartfield PW (1997) Status of aquatic mollusks in the southeastern United States: a downward spiral of diversity. In: Benz G, Colling D, editors. Aquatic Fauna in Peril: The Southeastern Perspective. Chattanooga, Tennessee: Southeast Aquatic Research Institute. [Google Scholar]
- 4. Johnson PD, Bogan AE, Brown KM, Burkhead NM, Cordeiro JR, et al. (2012) Conservation status of freshwater gastropods of Canada and the United States. Fisheries In Press. [Google Scholar]
- 5. Ó Foighil D, Li J, Lee T, Johnson PD, Evans RR, et al. (2011) Conservation genetics of a critically endangered limpet genus and rediscovery of an extinct species. PLoS ONE 6: e20496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Régnier C, Fontaine B, Bouchet P (2009) Not knowing, not recording, not listing: numerous unnoticed mollusk extinctions. Conservation Biology 23: 1214–1221. [DOI] [PubMed] [Google Scholar]
- 7.Turgeon DD, Quinn JF, Bogan AE, Coan EV, Hochberg FG, et al. (1998) Common and scientific names of aquatic invertebrates from the United States and Canada. Bethesda, MD: American Fisheries Society. [Google Scholar]
- 8. USFWS (1998) Final rule to list three aquatic snails as endangered and three aquatic snails as threatened in the Mobile River Basin of Alabama. Federal Register 63: 57610–57620. [Google Scholar]
- 9. USFWS (2010) Endangered and threatened wildlife and plants; determination of endangered status for the Georgia Pigtoe Mussel, Interrupted Rocksnail, and Rough Hornsnail and designation of critical habitat. Federal Register 75: 67512. [Google Scholar]
- 10.Johnson PD (2010) Proposed reintroduction of the Interrupted Rocksnail, Leptoxis foremani (Lea, 1843) into the Coosa River near Centre, Cherokee Country, Alabama. Final Report Submitted to the U.S. Fish and Wildlife Service 11. [Google Scholar]
- 11.Johnson PD (2010) Interim report and augmentation site plan for Leptoxis plicata, Plicate Rocksnail, in the Locust Fork of the Black Warrior River, Jefferson County, Alabama. Report Submitted to the Alabama Department of Conservation and Natural Resources 13. [Google Scholar]
- 12.Johnson PD, Evans RR (2001) The status of Leptoxis foremani in the upper Coosa River system of Georgia and Alabama. Report Submitted to the U.S. Fish and Wilflife Service 33. [Google Scholar]
- 13.Bogan AE (2000) Leptoxis compacta In: IUCN 2011. Red List of Threatened Species Version 2011.2. Available: http://www.iucnredlist.org. Accessed 2012 Jul 14.
- 14.Bogan AE, Pierson JM (1993) Survey of the aquatic gastropods of the Cahaba River Basin. Alabama: 1992. Final Report Submitted to the Alabama Department of Cosnervation and Natural Resources 20.
- 15. Johnson PD, Sides JD, McGregor SW, Ahlstedt SA, Garner JT, et al. (2006) Inventory of Freshwater Mollusks in the Cahaba River Basin, Alabama. Report Submitted to the Alabama Department of Conservation and Natural Resources 153. [Google Scholar]
- 16.Tolley-Jordan LR (2008) The biology of Pleuroceridae (Gatropoda: Caenogastropoda) in the Cahaba River Basin, Alabama. Tuscaloosa: Univeristy of Alabama Press. 239. [Google Scholar]
- 17. Goodrich C (1941) Distribution of the gastropods of the Cahaba River, Alabama. Occasional papers of the museum of zoology 428: 1–30. [Google Scholar]
- 18. Goodrich C (1922) The Anculosae of the Alabama River Drainage. University of Michigan Museum of Zoology Miscellaneous Publications 7: 63. [Google Scholar]
- 19. Shepard TE, O'Neil PE, McGregor SW, Harris SC (1994) Water-quality and biomonitoring studies in the upper Cahaba River drainage of Alabama. Geological Survey of Alabama Bulletin 160: 1–118. [Google Scholar]
- 20. Roberts DL, Elphick CS, Reed JM (2009) Identifying anomalous reports of putatively extinct species and why it matters. Conservation Biology 24: 189–196. [DOI] [PubMed] [Google Scholar]
- 21. Burch JB, Tottenham J (1980) North American freshwater snails, species list, ranges, and illustrations. Walkerana 3: 1–215. [Google Scholar]
- 22. Anthony JG (1854) Descriptions of new fluviatile shells of the genus Melania Lam., from western states of North America. Annals of the Lyceum of Natural History of New York 6: 122. [Google Scholar]
- 23. Minton RL, Garner JT, Lydeard C (2003) Rediscovery, systematic position, and re-description of “Leptoxis” melanoides (Conrad, 1834) (Mollusca: Gastropoda: Cerithioidea: Pleuroceridae) from the Black Warrior River, Alabama, U.S.A. Rediscovery and systematic position. Proceedings of the Biological Society of Washington 116: 531–541. [Google Scholar]
- 24. Minton RL (2002) A cladistic analysis of Lithasia (Gastropoda: Pleuroceridae) using morphological characters. The Nautilus 116: 39–49. [Google Scholar]
- 25.IUCN (2011) IUCN red list of threatened species. Version 2011.2. Available: http://www.iucnredlist.org. Accessed 2012 Jul 14..
- 26. Ahlstedt SA (1991) Reintroduction of the spiny riversnail Io Fluvialis (Say, 1825) (Gastropoda: Pleuroceridae) into the North fork Holston River, Southwest Virginia and Northeast Tennessee. American Malacological Bulletin 8: 139–142. [Google Scholar]
- 27.IUCN (1998) Guidelines for re-introcutions. Prepared by the IUCN/SSC reintroduction specialist group. Cambridge, UK: International Union for the Conservation of Nature and Natural Resources. Available: http://data.iucn.org/dbtw-wpd/edocs/PP-005.pdf. Accessed 2012 Jul 14.
- 28. George AL, Kuhajda BR, Williams JD, Cantrell MA, Rakes PL, et al. (2009) Guidelines for propagation and translocation for freshwater fish conservation. American Fisheries Society 34: 529–545. [Google Scholar]
- 29. Lysne SJ, Perez KE, Brown KM, Minton RL, Sides JD (2008) A review of freshwater gastropod conservation: challenges and opportunities. Journal of the North American Benthological Society 27: 463–470. [Google Scholar]
- 30.MRB Mollusc Recovery Committee (2010) Plan for the population restoration and conservation of imperiled freshwater mollusks of the Mobile River Basin. 106.
- 31. Huryn AD, Denny MW (1997) A biomechanical hypothesis explaining upstream movements by the freshwater snail Elimia . Functional Ecology 11: 472–483. [Google Scholar]
- 32. Kappes H, Haase P (2012) Slow, but steady: dispersal of freshwater molluscs. Aquatic Sciences 74: 1–14. [Google Scholar]
- 33. Vannote RL, Minshall GW, Cummins KW, Sedell JR, Cushing CE (1980) The river continuum concept. Canadian Journal of Fisheries and Aquatic Sciences 37: 130–137. [Google Scholar]
- 34.ADEM (2009) Final Total maximum daily load (TMDL) for Buck Creek assessment Unit ID # AL03150201-0202-101 pathogens (fecal coliform). Submitted to the Alabama Deparment of Environmental Management 45.
- 35.Howard HS, Quinn B, Flexner MC, Raschke RL (2002) Cahaba River: biological and water quality studies, Birmingham, Alabama. March/April, September and July, 2002. Athens, GA: US EPA Region 4 Science and Ecosystems Support Division. Available: http://www.epa.gov/region4/sesd/reports/2002-0809.html. Accessed 2012 Jul 14.
- 36. Seddon PJ, Armstrong DP, Maloney RF (2007) Developing the science of reintroduction biology. Conservation Biology 21: 303–312. [DOI] [PubMed] [Google Scholar]
- 37. Graf DL (2001) The cleanising of the augean stables or a lexicon of the nominal species of the Pleuroceridae (Gastropoda: Prosobranchia) of recent North American, North of Mexico. Walkerana 12: 1–124. [Google Scholar]
- 38. Fukuda H, Haga T, Tatara Y (2008) Niku-nuki: a useful method for anatomical and DNA studies on shell bearing molluscs. Zoosymposia 1: 15–28. [Google Scholar]
- 39. Holznagel WE (1997) A nondestructive method for cleaning gastropod radulae from frozen, alcohol-fixed or dried material. American Malacological Bulletin 14: 181–184. [Google Scholar]
- 40. Whelan NV, Johnson PD, Harris PM (2012) Presence or absence of carinae in closely related populations of Leptoxis ampla (Anthony, 1855) (Gastropoda: Cerithioidea: Pleuroceridae) is not the result of ecophenotypic plasticty. Journal of Molluscan Studies 78: 231–233. [Google Scholar]