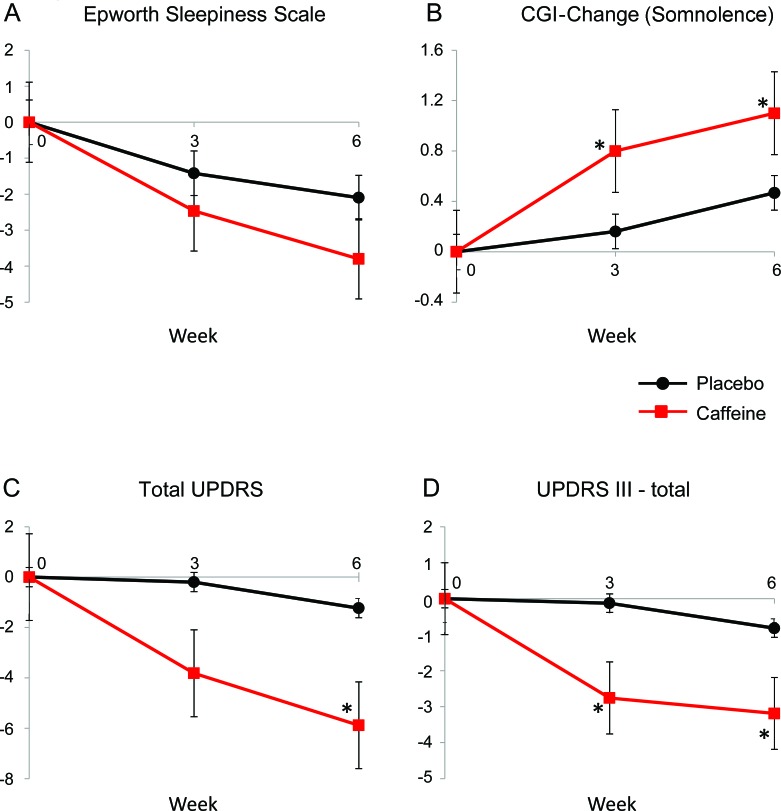
Figure 2. Change in outcomes in caffeine vs placebo.
(A) Epworth Sleepiness Scale, (B) Clinical Global Impression (CGI)–Change, (C) total Unified Parkinson's Disease Rating Scale (UPDRS), (D) UPDRS part III. Shown are the changes in major outcomes of interest in caffeine and placebo over the 6-week trial. Caffeine dose at week 3 = 100 mg BID, and at week 6 = 200 mg BID. Baseline values are set at 0. Error bars indicate standard error. * Significant difference from placebo, p < 0.05.