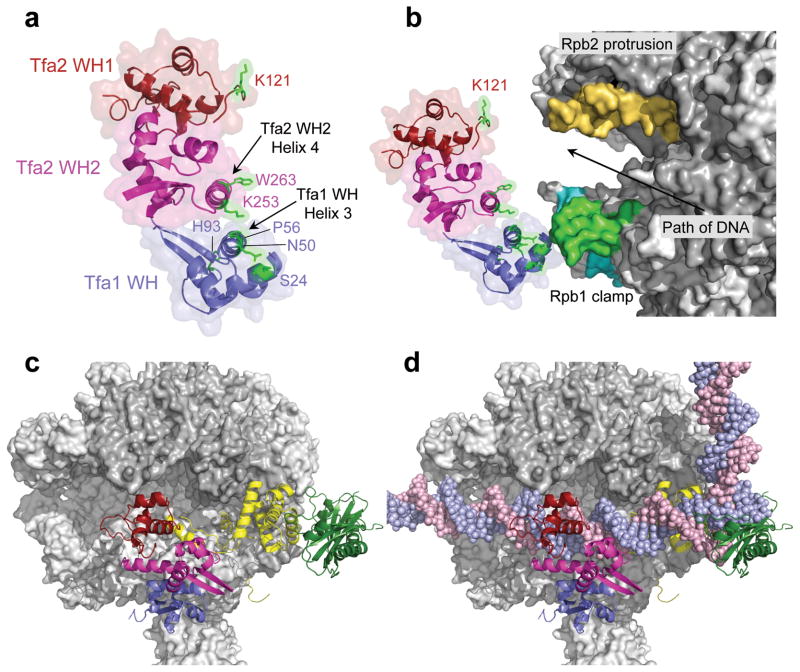
Figure 4.
Position of TFIIE in the PIC. (a) The TFIIE dimerization domain is formed in part by helix 3 in the Tfa1 WH domain and helix 4 in the Tfa2 WH2 domain. FeBABE-positions that cleave Pol II are highlighted in green. The Tfa1 WH domain is colored blue; the Tfa2 WH1 and WH2 domains are colored red and pink, respectively. (b) Position of the three TFIIE WH domains in the PIC based on the combined FeBABE cleavage data. Residues in TFIIE that cleave Pol II when linked to FeBABE are highlighted in green, as is the corresponding cleavage site in the coiled-coil region of the Pol II clamp. The cleavage site in the clamp head by Tfa1 residue His93 is colored in cyan, and cleavage in the Rpb2 protrusion domain is colored orange. An arrow indicates the path of the double stranded DNA in the PIC. (c, d) Top view of the Pol II PIC model containing Pol II (grey), TFIIB (yellow), TBP (dark green), and TFIIE without (c) and with (d) promoter DNA.