Figure 5.
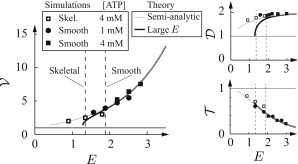
Comparison between theoretical calculations and simulations for actin speed (), myosin attachment time (), and attachment distance (), all normalized to the single-molecule values (single-molecule values are shown as a thin, solid line). These plots are shown as a function of the nondimensional mechanochemical coupling parameter E. Simulations were performed with large ensembles (N = 400) with kinetic parameters based on smooth muscle and skeletal muscle. The value E was varied by using different myosin stiffness values and/or force dependence. (Dashed lines) Our best estimate of E. Theoretical calculations, described in the text and the Supporting Material, agree with the simulations.
