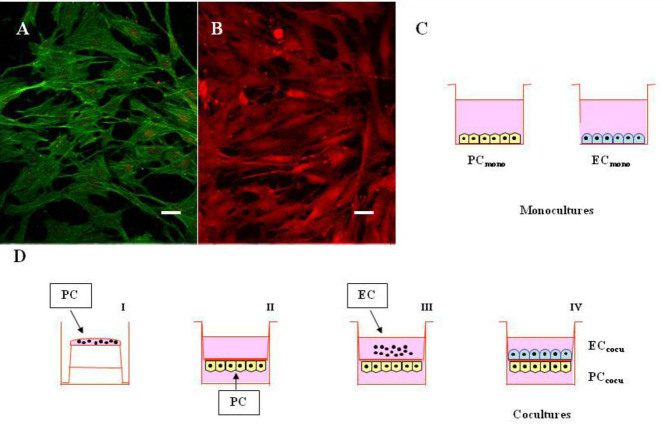
Figure 1.
Monocultures of pericytes and endothelial cells, and cocultures with direct cell-to-cell contact. A-B: Confocal fluorescence representative images of retinal pericytes and endothelial cells in coculture. A: Pericytes monolayer was stained with a monoclonal anti-α-actin antibody coupled to a green fluorescent protein-labeled fluorescein isothiocyanate secondary antibody. B: Endothelial cells monolayer was stained with a polyclonal anti-von Willebrand factor antibody coupled to a red fluorescent protein-labeled Cy3 secondary antibody. Scale bars: 20 μm. C-D: Schematic representation of mono- and cocultures. (C) Pericytes and endothelial cells were cultured in monolayers (PCmono and ECmono, respectively), and incubated with or without 100 nM PMA for 15 min. D: Pericytes (PC) were first plated on the outside of the membrane filter (40,000 cells; I) and, 4 h after, the inserts were turned upside down and placed into the six-well plates with the culture medium (II). Endothelial cells (EC) were plated on the top surface of the same inserts (40,000 cells) on which PC had been plated 2 days before (III). In this system, cells on both sides of the insert are exposed to the same conditioned medium (IV), and incubated with or without 100 nM PMA for 15 min. The pore size (0.4 μm) of the membrane filter was chosen to avoid the passage of cell foot processes through the membrane filter separating the upper and lower chambers.