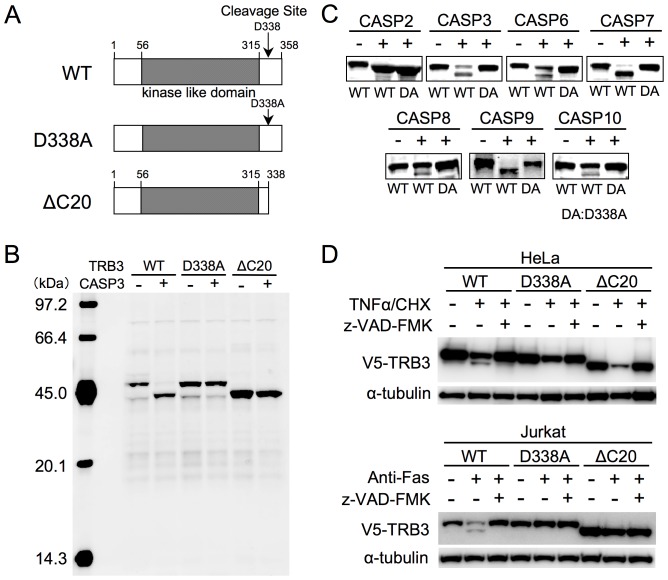
Figure 1. TRB3 is cleaved by caspases in vitro and in the apoptotic process.
(A) Schematic representation of proteins expressed by the recombinant TRB3 constructs used in this study. WT, wild type TRB3; D338A, CASP3 cleavage-site mutant TRB3; and ΔC20, CASP3-cleaved form of TRB3. (B and C) Each recombinant TRB3 was synthesized and biotinylated using the wheat cell-free expression system as described in the Materials and Methods. The translation mixture was incubated with or without the indicated active caspase for 2 hr at 30°C. For the detection of N-terminal biotinylated-TRB3, the blot was probed with Alexa 488-conjugated streptavidin. DA denotes D338A-TRB3. (D) Twenty-four hours after transfection of HeLa and Jurkat cells with the indicated V5-tagged TRB3 expression plasmid, cells were treated with TNFα (20 ng/mL)/CHX (100 µM) (HeLa cells) or anti-Fas antibody (125 ng/mL) (Jurkat cells) in the absence or presence of z-VAD-FMK (100 µM) for 4 hr. DMSO was used as a treatment control. The cell lysates were subjected to immunoblot analysis using anti-V5 antibody to detect the N-terminal V5-tagged TRB3. α-Tubulin was used as an internal control.